Abstract
Background
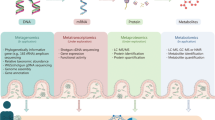
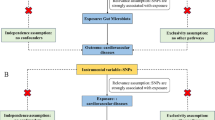
The pathogenesis of cardiac arrhythmias is multifaceted, encompassing genetic, environmental, hemodynamic, and various causative factors. Emerging evidence underscores a plausible connection between gut flora, serum metabolites, and specific types of arrhythmias. Recognizing the role of host genetics in sha** the microbiota, we employed two-sample Mendelian randomization analyses to investigate potential causal associations between gut flora, serum metabolites, and distinct arrhythmias.
Methods
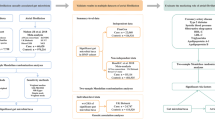
Mendelian randomization methods were deployed to ascertain causal relationships between 211 gut flora, 575 serum metabolites, and various types of arrhythmias. To ensure the reliability of the findings, five complementary Mendelian randomization methods, including inverse variance weighting methods, were employed. The robustness of the results was scrutinized through a battery of sensitivity analyses, incorporating the Cochran Q test, leave-one-out test, and MR-Egger intercept analysis.
Results
Eighteen gut flora and twenty-six serum metabolites demonstrated associations with the risk of develo** atrial fibrillation. Moreover, ten gut flora and fifty-two serum metabolites were linked to the risk of develo** supraventricular tachycardia, while eight gut flora and twenty-five serum metabolites were associated with the risk of develo** tachycardia. Additionally, six gut flora and twenty-one serum metabolites exhibited associations with the risk of develo** bradycardia.
Conclusion
This study revealed the potential causal relationship that may exist between gut flora, serum metabolites and different cardiac arrhythmias and highlights the need for further exploration. This study provides new perspectives to enhance diagnostic and therapeutic strategies in the field of cardiac arrhythmias.
Similar content being viewed by others
Introduction
Arrhythmia is a disorder of the origin and/or conduction of cardiac activity resulting in an abnormal rate and/or rhythm of the heartbeat, which may result in sudden death from a sudden onset, or may continue to involve the heart and cause it to fail [1,2,3]. Arrhythmias can be categorized into bradyarrhythmias and tachyarrhythmias according to the heart rate, with bradyarrhythmias mainly characterized by bradycardia and atrioventricular block, and tachyarrhythmias mainly characterized by tachycardia, supraventricular tachycardia, ventricular fibrillation, and atrial fibrillation [4]. Atrial fibrillation causes hemodynamic abnormalities and increases the morbidity and mortality of thromboembolic events, while ventricular arrhythmias can lead to palpitations or blackouts and even sudden cardiac death, as all types of arrhythmias add to the global economic burden [5,6,7].
Gut flora is a group of microorganisms that are planted in the human intestinal tract and are interdependent with the human body over a long period of time, which is very large in number and is known as the "second genome" of the human body [1) [10, 21]. Data from SNPs with chained unbalanced aggregates were subsequently removed, with removal conditioned on LD (r2 < 0.001, distance = 10,000 kb). SNPs that did not belong to a specific bacterial trait were excluded. Serum metabolite data were downloaded from the GWAS data, and a total of 575 metabolite-associated exposures were collected, and SNPs were screened based on P < 1 × 10–5, r2 < 0.001, distance < 10 000 kb (Supplementary Table S2) [22].
Outcome sources
Data on atrial fibrillation were derived from a GWAS of susceptibility genes published in 2018 (PMID: 30,061,737), the study included 60,620 patients with atrial fibrillation, 970,216 healthy controls, and contained 33,519,037 SNPs [23]. Data for the remaining types of arrhythmic disease were obtained from studies in the UK Biobank and Finnish databases, with studies of supraventricular tachycardia comprising 1,306 patients, 461,704 healthy controls, and 9,851,867 SNPs. studies of bradycardia comprised 1,005 patients with supraventricular tachycardia, 462,005 healthy controls, and 9,851,867 SNPs. studies of bradycardia comprised 1,254 patients, 461,756 healthy controls, and 9,851,867 SNPs. The study of bradycardia contained 1,254 patients, 461,756 healthy controls, and 9,851,867 SNPs. The study of atrioventricular block contained 5,536 patients, 286,109 healthy controls, and 16,380,173 SNPs. The study of left bundle branch block consisted of 1,918 patients, 286,109 healthy controls, and 16,380,167 SNPs. The study of right bundle branch block consisted of 9,545 patients, 286,109 healthy controls, and 16,380,175 SNPs. The study of right bundle branch block consisted of 9,545 patients, 286,109 healthy controls, and 16,380,175 SNPs (Table 1). In this study, the diagnostic criteria for all types of arrhythmias adhere to the International Classification of Diseases, Tenth Revision (ICD-10).
Statistical methods
In this study, the association between gut flora and various types of arrhythmias was analyzed by inverse variance weighting (IVW). The mean IVW values of SNP ratio estimates were derived by regressing SNP—gut flora on SNP—arrhythmia associations. The weighted median method (WME), MR-Egger regression test, Simple mode, and Weighted mode were used as supplements. Cochrane's Q was used to test the snp-related heterogeneity of each bacterial trait. In addition, sensitivity analyses were performed using the MR-Egger intercept test and leave-one-out analysis. p-values from the MR-Egger intercept test were used as an indicator of horizontal pleiotropy (p < 0.05 statistically significant). Leave-one-out analysis was used to identify potential pleiotropic effects from individual SNPs.
Results
Causal relationship between gut flora and various types of cardiac arrhythmias
Causal relationship between gut flora and atrial fibrillation
IVW analysis showed that family Family XIII id.1957 (OR = 0.87, 95% CI: 0.80-0.0.94, p = 0.0005) and phylum Lentisphaerae id.2238 (OR = 0.93, 95% CI: 0.89–0.97, p = 0.0007) and other 10 gut flora were negatively associated with the development of atrial fibrillation. genus Lachnospiraceae FCS020 group id.11314 (OR = 1.08, 95% CI: 1.01–1.15, p = 0.0209) and genus Streptococcus id.1853 (OR = 1.09, 95% CI: 1.01–1.18, p = 0.0299) and 4 other gut flora were positively associated with the development of atrial fibrillation (Supplementary Table S3) (Fig. 2A).
Causal relationship between gut flora and various types of cardiac arrhythmias. A Causal relationship between gut flora and atrial fibrillation. B Causal relationship between metabolites and supraventricular tachycardia. C Causal relationship between gut flora and tachycardia. D Causal relationship between gut flora and bradycardia. E Causal relationship between gut flora and atrioventricular block. F Causal relationship between gut flora and left bundle branch block
Causal relationship between gut flora and supraventricular tachycardia
IVW analysis showed that Family XIII id.1957 (OR = 0.99, 95% CI: 1.00–1.00, p = 0.0099) and phylum Lentisphaerae id.2238 (OR = 0.99, 95% CI: 0.99–1.00, p = 0.01659) and other 8 gut flora were positively associated with the development of supraventricular tachycardia (Supplementary Table S4) (Fig. 2B).
Causal relationship between gut flora and tachycardia
IVW analysis showed that 8 species of gut flora including family Desulfovibrionaceae id.3169 (OR = 0.99, 95% CI: 0.99–1.00, p = 0.0040) and genus Ruminococcaceae UCG013 id.11370 (OR = 1.01, 95% CI:1.00- 1.01, p = 0.0193) were positively associated with the development of tachycardia (Supplementary Table S5) (Fig. 2C).
Causal relationship between gut flora and bradycardia
IVW analysis showed that 6 gut flora including genus Christensenellaceae R 7group id.11283 (OR = 0.99, 95% CI: 0.99–0.99, p = 0.0096) and genus Escherichia Shigella id.3504 (OR = 1.00, 95% CI. 1.00–1.00, p = 0.0210) were positively associated with the development of bradycardia (Supplementary Table S6) (Fig. 2D).
Causal relationship between gut flora and atrioventricular block
IVW analysis showed that 2 gut flora, including genus Lachnospira id.2004 (OR = 0.56, 95% CI: 0.38–0.81, p = 0.0024) and genus Clostridium sensustricto1 id.1873 (OR = 0.61, 95% CI: 0.40–0.93, p = 0.0210) (Supplementary Table S7) (Fig. 2E).
At the same time, we found that the causal relationship between gut flora and the organization of left bundle branch block and atrioventricular block obtained by our analysis was almost the same(Supplementary Table S8) (Fig. 2F).
Causal relationship between metabolites and various types of arrhythmias
Causal relationship between metabolites and atrial fibrillation
IVW analysis showed that 12 metabolites, including Tryptophan betaine (OR = 0.83, 95% CI: 0.76–0.90, p = 0.0001) and Uridine (OR = 0.58, 95% CI: 0.40–0.84, p = 0.0037) were negatively associated with the development of AF. 14 metabolites including Docosahexaenoate (DHA; 22:6n3) (OR = 1.33, 95% CI:1.04–1.70, p = 0.0252) and Carnitine (OR = 1.31, 95% CI:1.02–1.69, p = 0.0348), were positively associated with the onset of AF (Supplementary Table S9) (Fig. 3A).
Causal relationship between metabolites and supraventricular tachycardia
IVW analysis showed that 3 metabolites, Isobutyrylcarnitine (OR = 0.99, 95% CI: 0.99–1.00, p = 0.0003) and Pipecolate (OR = 0.99, 95% CI: 0.99–1.00, p = 0.0275) were negatively associated with the development of supraventricular tachycardia. Triglycerides in large VLDL (OR = 1.33, 95% CI: 1.00–1.00, p = 0.0131) and total cholesterol in small LDL (OR = 1.00, 95% CI: 1.00–1.00, p = 0.0016) and 47 other metabolites were positively associated with the development of supraventricular tachycardia (Supplementary Table S10) (Fig. 3B).
Causal relationship between metabolites and tachycardia
IVW analysis showed that 4 metabolites, 1-palmitoleoylglycerophosphocholine (OR = 0.99, 95% CI:0.99–1.00, p = 0.0217) and gamma-glutamylisoleucine (OR = 0.99, 95% CI:0.98–1.00, p = 0.0477) were negatively associated with the onset of tachycardia. N2-dimethylguanosine (OR = 1.01, 95% CI:1.00–1.00, p = 0.0376) and X-04499–3,4-dihydroxybutyrate (OR = 1.01, 95% CI: 1.00–1.02, p = 0.0127) and 19 other metabolites were positively associated with the development of tachycardia (Supplementary Table S11) (Fig. 4A).
Causal relationship between metabolites and bradycardia
IVW analysis showed that 4 metabolites, 2-stearoylglycerophosphocholine (OR = 0.99, 95% CI:0.99–1.00, p = 0.0051) and Serotonin (5HT) (OR = 0.99, 95% CI:0.99–1.00, p = 0.0072) were negatively associated with the onset of bradycardia. Erythronate (OR = 1.01, 95% CI: 1.00–1.01, p = 0.0438) and 1-arachidonoylglycerophosphoinositol (OR = 1.01, 95% CI: 1.00–1.01, p = 0.0471) and 15 other metabolites were positively associated with the development of tachycardia (Supplementary Table S12) (Fig. 4B).
Causal relationship between metabolites and atrioventricular block
IVW analysis showed that X-12230 (OR = 0.41, 95% CI: 0.18–0.93, p = 0.0338) was negatively associated with the onset of atrioventricular block, and no metabolite was positively associated with the onset of atrioventricular block (Supplementary Table S13) (Fig. 4C).
Sensitivity analysis
Heterogeneity test: the results of the Q-test showed no heterogeneity between the included SNPs (p > 0.05). Horizontal pleiotropy: The results of MR-Egger regression intercepts showed no horizontal pleiotropy in the associations of gut flora and serum metabolites with the associations with each type of arrhythmia. The absence of SNPs with large effects on effect estimates in the analyses of gut flora and serum metabolites with each type of arrhythmia suggests that a causal relationship exists and that the causal relationship is reasonably stable.
Discussion
There is already a lot of research supporting the theory of a "gut-heart" axis-centred relationship between gut microbes and heart health, which means that the gut flora can influence the host's metabolism, inflammation levels, and immune system, which ultimately affects the heart's health [29,30,31]. Another study found that the abundance of Bifidobacterium and Ruminococcaceae were inversely related to different markers of low-grade inflammation such as hsCRP and interleukin (IL)-6, and Ruminococcaceae ruminantium reduces the inflammatory response by modulating T cell numbers and producing short-chain fatty acids [32, 33]. Therefore, we hypothesised that intestinal flora such as Bifidobacterium and Ruminococcaceae may reduce the risk of AF by suppressing the inflammatory response. Our study identified Clostridium lachnospira and Clostridium sensustricto, bacteria known for their ability to break down carbohydrates. Chronic consumption of high-sugar carbohydrates may lead to insulin resistance, which is associated with the development of chronic inflammation, and therefore we hypothesise that these two bacteria may influence the risk of develo** left bundle branch block and atrioventricular block through this mechanism. Additionally, our study identified anaerobic bacteria like Lachnospira and Rikenellaceae, which may be linked to an increased risk of arrhythmia These anaerobic bacteria break down carbohydrates and produce short-chain fatty acids (e.g., acetic acid, propionic acid, and butyric acid) and alcohols (ethanol, isopropanol, and butanol), which affect the activity of immune cells and inflammatory responses, and the various alcohols they produce are metabolised by alcohol, causing cardiomyocyte damage and death, which ultimately leads to alterations in cardiac structure and function [34, 34, 38, 39]. Immunocytes such as macrophages are normally present in large numbers right in the heart, and the inflammatory response can regulate calcium homeostasis and connexins through pathways such as CXCR4 and TYROBP and cause changes in atrial electrophysiology and structural substrates, as well as affecting the resting membrane potential and action potential of cardiomyocytes [40,41,42]. Flora such as Bifidobacterium and Lactobacillus were identified in this study, which have been shown to be involved in modulating the immune response and may therefore influence the risk of arrhythmia development from this mechanism [43, 44].
Subsequently, we co-analysed the MR results of serum metabolomics with those of intestinal flora and found similarities between the trends of some intestinal flora and those of serum metabolites, which may collectively affect the risk of arrhythmia development. The heart is controlled by the autonomic nervous system, and a variety of neurotransmitters affect the electrophysiological properties of cardiac cells, including the duration and conduction velocity of action potentials. The present study found that serum metabolites such as tryptophan, isoleucine and valine may be closely associated with the risk of AF. Branched-chain amino acids such as tryptophan are one of the raw materials for synthesising neurotransmitters and it has been demonstrated that there is a close correlation between the relationship between elevated branched-chain amino acids and cardiac arrhythmias, so our study further provides some theoretical support for this conclusion [45]. At the same time, metabolites such as tryptophan are intricately linked to inflammatory regulation and immune modulation, as part of the metabolism of tryptophan takes place in the gut [46]. In the results of previous MR gut flora analyses, Bifidobacteria, Anaerobacteria, and Odorobacteria were found to be potentially protective against certain types of cardiac arrhythmias, and all of these flora were shown to have a strong relationship with tryptophan metabolism [40]. In addition, our analyses revealed that uridine may be strongly associated with the risk of develo** AF. It has been suggested that uridine may modulate the inflammatory response by inhibiting inflammatory cell activity and other pathways, but whether it affects the risk of develo** AF through this pathway deserves further exploration [47, 48]. Lactate and valine are both common serum metabolites that were similarly found in this study to potentially influence the risk of develo** atrial fibrillation. It has been demonstrated that lactate accelerates vascular calcification and leads to mitochondrial dysfunction, and that valine exhibits antiarrhythmic effects in ischaemia–reperfusion experiments, mechanisms of action that may be closely related to cardiomyocyte apoptosis and inflammatory responses [49,50,51]. Meanwhile, combined with the results of our intestinal flora MR analyses, it was demonstrated that flora such as Bactericide and genus Odoribacter are strongly associated with lactate and valine, but the mechanism of action in disease remains unclear [52]. In addition, we have identified serum metabolites that may be associated with the risk of develo** supraventricular tachycardia, bradycardia, tachypnea, and atrioventricular block, but the effects may not be as closely related as those of atrial fibrillation.
Prospects and limitations of clinical applications
Based on the aforementioned research findings, the potential roles of gut microbiota and serum metabolites in arrhythmias reveal promising prospects for future clinical applications. Initially, alterations in specific gut microbiota and serum metabolites may serve as biomarkers for the early detection of arrhythmias. Furthermore, understanding how particular gut microbiota influence arrhythmias can aid in the development of personalized treatment plans. For instance, modifying one's diet to alter microbial composition, such as increasing probiotics, prebiotics, or certain types of fiber, might reduce the risk of atrial fibrillation if an individual's mouth and gut flora indicates a higher predisposition [53, 54]. Additionally, since gut microbiota can affect inflammation and immune responses through various mechanisms, they might present novel therapeutic targets for arrhythmia management. Despite this field offering many promising directions, current research is primarily still in the exploratory stage.
Limitations of this study include: Firstly, the research data encompasses only subjects of European descent, potentially limiting applicability to other populations. Secondly, although this is the first use of MR to investigate potential causal relationships between gut microbiota, metabolomics, and various types of arrhythmias, most conclusions are still theoretical. Further extensive research is required to establish specific causal links, evaluate intervention effectiveness, and consider inter-individual differences. Lastly, some gut microbiota and serum metabolites are still in the naming phase, with specific bacteria not yet definitively identified.
Conclusion
Gut flora and serum metabolites may influence the risk of develo** arrhythmias, but finding key gut flora and serum metabolites and exploring their specific mechanisms still requires extensive experimental validation.
Availability of data and materials
Publicly available datasets were analyzed in this study. This data can be found at: https://mibiogen.gcc.rug.nl/, https://r7.finngen.fi/, and https://gwas.mrcieu.ac.uk./
References
Guasch E, Mont L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Nat Rev Cardiol. 2017;14(2):88–101.
Marcus FI, Ruskin JN, Surawicz B. Cardiovascular disease in the elderly. Arrhythmias. J Am Coll Cardiol. 1987;10(2 Suppl A):66A-72A.
George AL Jr. Molecular and genetic basis of sudden cardiac death. J Clin Invest. 2013;123(1):75–83.
Evolution in the treatment of arrhythmias. 22–23 September 1983, Brighton, U.K. Eur Heart J 1984, 5 Suppl B:1–137.
Narayan SM, Cain ME, Smith JM. Atrial fibrillation. Lancet. 1997;350(9082):943–50.
Griffith KE, Pye M. Atrial fibrillation. BMJ. 2008;337:a475.
Morin DP, Estes NA 3rd. Advances in the prevention and treatment of atrial fibrillation. Prog Cardiovasc Dis. 2015;58(2):103–4.
Zhou B, Yuan Y, Zhang S, Guo C, Li X, Li G, **ong W, Zeng Z. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front Immunol. 2020;11:575.
Carmody RN, Sarkar A, Reese AT. Gut microbiota through an evolutionary lens. Science. 2021;372(6541):462–3.
Zeng Y, Cao S, Yang H. Roles of gut flora in epilepsy risk: A Mendelian randomization study. Front Microbiol. 2023;14:1115014.
Fang C, Zuo K, Liu Z, Liu Y, Liu L, Wang Y, Yin X, Li J, Liu X, Chen M, et al. Disordered gut microbiota promotes atrial fibrillation by aggravated conduction disturbance and unbalanced linoleic acid/SIRT1 signaling. Biochem Pharmacol. 2023;213:115599.
Holmes D. Gut microbiota: Antidiabetic drug treatment confounds gut dysbiosis associated with type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12(2):61.
Rashid S, Noor TA, Saeed H, Ali AS, Meheshwari G, Mehmood A, Fatima L, Zaidi SMJ, Malik J, Mehmoodi A, et al. Association of gut flora dysbiosis with the progression of atrial fibrillation: a systematic review. Ann Noninvasive Electrocardiol. 2023;28(4):e13059.
Palmu J, Borschel CS, Ortega-Alonso A, Marko L, Inouye M, Jousilahti P, Salido RA, Sanders K, Brennan C, Humphrey GC, et al. Gut flora and atrial fibrillation-results from a large population-based study. EBioMedicine. 2023;91:104583.
Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J Am Soc Mass Spectrom. 2016;27(12):1897–905.
Bauermeister A, Mannochio-Russo H, Costa-Lotufo LV, Jarmusch AK, Dorrestein PC. Mass spectrometry-based metabolomics in flora investigations. Nat Rev Microbiol. 2022;20(3):143–60.
Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17(7):451–9.
Li Y, Fu R, Li R, Zeng J, Liu T, Li X, Jiang W. Causality of gut flora and hypertension: a bidirectional mendelian randomization study. Front Cardiovasc Med. 2023;10:1167346.
Zeng Y, Cao S, Yang H. Circulating sex hormone-binding globulin levels and ischemic stroke risk: a Mendelian randomization study. Postgrad Med J. 2023;99:1272–9.
Yin Z, Liu B, Feng S, He Y, Tang C, Chen P, Wang X, Wang K. A Large genetic causal analysis of the gut microbiota and urological cancers: a bidirectional mendelian randomization study. Nutrients. 2023;15(18):4086.
Zhang L, Zi L, Kuang T, Wang K, Qiu Z, Wu Z, Liu L, Liu R, Wang P, Wang W. Investigating causal associations among gut microbiota, metabolites, and liver diseases: a Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1159148.
Liu X, Tong X, Zou Y, Lin X, Zhao H, Tian L, Jie Z, Wang Q, Zhang Z, Lu H, et al. Mendelian randomization analyses support causal relationships between blood metabolites and the gut flora. Nat Genet. 2022;54(1):52–61.
Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50(9):1234–9.
Huang Y, **n W, **ong J, Yao M, Zhang B, Zhao J. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front Pharmacol. 2022;13:837500.
Zhang Y, Zhang S, Li B, Luo Y, Gong Y, ** X, Zhang J, Zhou Y, Zhuo X, Wang Z, et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc Res. 2022;118(3):785–97.
Troseid M, Andersen GO, Broch K, Hov JR. The gut flora in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine. 2020;52:102649.
Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res. 2017;120(9):1501–17.
Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–68.
Abdi M. Esmaeili Gouvarchin Ghaleh H, Ranjbar R: Lactobacilli and Bifidobacterium as anti-atherosclerotic agents. Iran J Basic Med Sci. 2022;25(8):934–46.
Nam HS. Gut Microbiota and Ischemic Stroke: The Role of Trimethylamine N-Oxide. J Stroke. 2019;21(2):151–9.
Svingen GFT, Zuo H, Ueland PM, Seifert R, Loland KH, Pedersen ER, Schuster PM, Karlsson T, Tell GS, Schartum-Hansen H, et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int J Cardiol. 2018;267:100–6.
van den Munckhof ICL, Kurilshikov A, Ter Horst R, Riksen NP, Joosten LAB, Zhernakova A, Fu J, Keating ST, Netea MG, de Graaf J, et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev. 2018;19(12):1719–34.
Ma F, Sun M, Song Y, Wang A, Jiang S, Qian F, Mu G, Tuo Y. Lactiplantibacillus plantarum-12 Alleviates Inflammation and Colon Cancer Symptoms in AOM/DSS-Treated Mice through Modulating the Intestinal Flora and Metabolome. Nutrients. 2022;14(9):1916.
El-Mas MM, Abdel-Rahman AA. Role of Alcohol Oxidative Metabolism in Its Cardiovascular and Autonomic Effects. Adv Exp Med Biol. 2019;1193:1–33.
Liang Y, Xu X, Li Q, Deng Y, **e M, Zheng Y, Ou W, He Q, Xu X, Wu W, et al. Chronic Alcohol Intake Exacerbates Cardiac Dysfunction After Myocardial Infarction. Alcohol Alcohol. 2020;55(5):524–30.
Fan H, Liu X, Ren Z, Fei X, Luo J, Yang X, Xue Y, Zhang F, Liang B. Gut microbiota and cardiac arrhythmia. Front Cell Infect Microbiol. 2023;13:1147687.
Gawalko M, Agbaedeng TA, Saljic A, Muller DN, Wilck N, Schnabel R, Penders J, Rienstra M, van Gelder I, Jespersen T, et al. Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc Res. 2022;118(11):2415–27.
Naik SS, Ramphall S, Rijal S, Prakash V, Ekladios H, Mulayamkuzhiyil Saju J, Mandal N, Kham NI, Shahid R, Venugopal S. Association of Gut Microbial Dysbiosis and Hypertension: A Systematic Review. Cureus. 2022;14(10):e29927.
Wang Z, Hazen J, Jia X, Org E, Zhao Y, Osborn LJ, Nimer N, Buffa J, Culley MK, Krajcik D, et al. The Nutritional Supplement L-Alpha Glycerylphosphorylcholine Promotes Atherosclerosis. Int J Mol Sci. 2021;22(24):13477.
Fang H, Fang M, Wang Y, Zhang H, Li J, Chen J, Wu Q, He L, Xu J, Deng J, et al. Indole-3-Propionic Acid as a Potential Therapeutic Agent for Sepsis-Induced Gut Microbiota Disturbance. Microbiol Spectr. 2022;10(3):e0012522.
Liu G, Lu J, Sun W, Jia G, Zhao H, Chen X, Kim IH, Zhang R, Wang J. Tryptophan Supplementation Enhances Intestinal Health by Improving Gut Barrier Function, Alleviating Inflammation, and Modulating Intestinal Flora in Lipopolysaccharide-Challenged Piglets. Front Microbiol. 2022;13:919431.
Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):120.
Moawad MH, Alkhawaldeh IM, Naswhan AJ. Efficacy of probiotics supplementation in amelioration of celiac disease symptoms and enhancement of immune system. World J Clin Cases. 2023;11(32):7741–4.
Li X, Hu S, Yin J, Peng X, King L, Li L, Xu Z, Zhou L, Peng Z, Ze X, et al. Effect of synbiotic supplementation on immune parameters and gut microbiota in healthy adults: a double-blind randomized controlled trial. Gut Microbes. 2023;15(2):2247025.
Portero V, Nicol T, Podliesna S, Marchal GA, Baartscheer A, Casini S, Tadros R, Treur JL, Tanck MWT, Cox IJ, et al. Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc Res. 2022;118(7):1742–57.
Su X, Gao Y, Yang R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells. 2022;11(15):2296.
Lai K, Song C, Gao M, Deng Y, Lu Z, Li N, Geng Q. Uridine Alleviates Sepsis-Induced Acute Lung Injury by Inhibiting Ferroptosis of Macrophage. Int J Mol Sci. 2023;24(6):5093.
Zhang L, Li B, Zhang D, Wang Z, Zhao Y, Yu Q. Uridine alleviates LPS-induced ARDS and improves insulin sensitivity by decreasing oxidative stress and inflammatory processes. Physiol Int. 2022;109(2):215–29.
** C, Yuan S, Piao L, Ren M, Liu Q. Propofol synergizes with circAPBB2 to protect against hypoxia/reoxygenation-induced oxidative stress, inflammation, and apoptosis of human cardiomyocytes. Immun Inflamm Dis. 2023;11(8):e952.
Zhu Y, Zhang JL, Yan XJ, Ji Y, Wang FF. Exploring a new mechanism between lactate and VSMC calcification: PARP1/POLG/UCP2 signaling pathway and imbalance of mitochondrial homeostasis. Cell Death Dis. 2023;14(9):598.
Mitrega K, Zorniak M, Varghese B, Lange D, Nozynski J, Porc M, Bialka S, Krzeminski TF. Beneficial effects of l-leucine and l-valine on arrhythmias, hemodynamics and myocardial morphology in rats. Pharmacol Res. 2011;64(3):218–25.
Shetty SA, Boeren S, Bui TPN, Smidt H, de Vos WM. Unravelling lactate-acetate and sugar conversion into butyrate by intestinal Anaerobutyricum and Anaerostipes species by comparative proteogenomics. Environ Microbiol. 2020;22(11):4863–75.
Nalliah CJ, Sanders P, Kalman JM. The Impact of Diet and Lifestyle on Atrial Fibrillation. Curr Cardiol Rep. 2018;20(12):137.
Tierney BT, Yang Z, Luber JM, Beaudin M, Wibowo MC, Baek C, Mehlenbacher E, Patel CJ, Kostic AD. The Landscape of Genetic Content in the Gut and Oral Human Flora. Cell Host Microbe. 2019;26(2):283-295 e288.
Acknowledgements
We acknowledge the participants and investigators of all studys.
Funding
None.
Author information
Authors and Affiliations
Contributions
KL and PL designed the study, analyzed the data, and wrote the manuscript. ML, JY and LZ revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Publicly available de-identified data from participant studies approved by an ethical standards committee were used in this study. Therefore, no additional separate ethical approval was required for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
2,232 SNPs in the gut flora were identified as Final IV.
Additional file 2: Supplementary table 2.
10,075 SNPs in serum metabolites were identified as Final IV.
Additional file 3: Supplementary Table S3.
Causal relationship between gut flora and atrial fibrillation.
Additional file 4: Supplementary Table S4.
Causal relationship between gut flora and supraventricular.
Additional file 5: Supplementary Table S5.
Causal relationship between gut flora and tachycardia.
Additional file 6: Supplementary Table S6.
Causal relationship between gut flora and bradycardia.
Additional file 7: Supplementary Table S7.
Causal relationship between gut flora and atrioventricular block.
Additional file 8: Supplementary Table S8.
Causal relationship between gut flora and left bundle branch block.
Additional file 9: Supplementary Table S9.
Causal relationship between metabolites and atrial fibrillation.
Additional file 10: Supplementary Table S10.
Causal relationship between metabolites and supraventricular tachycardia.
Additional file 11: Supplementary Table S11.
Causal relationship between metabolites and tachycardia.
Additional file 12: Supplementary Table S12.
Causal relationship between metabolites and bradycardia.
Additional file 13: Supplementary Table S13.
Causal relationship between metabolites and atrioventricular block.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, K., Liu, P., Liu, M. et al. Putative causal relations among gut flora, serums metabolites and arrhythmia: a Mendelian randomization study. BMC Cardiovasc Disord 24, 38 (2024). https://doi.org/10.1186/s12872-023-03703-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03703-z