Abstract
Background
Patients with COVID-19 undergoing pressure support ventilation (PSV) with extracorporeal membrane oxygenation (ECMO) commonly had high respiratory drive, which could cause self-inflicted lung injury. The aim of this study was to evaluate the influence of different levels of partial pressure of carbon dioxide(PaCO2) on respiratory effort in COVID-19 patients undergoing PSV with ECMO.
Methods
ECMO gas flow was downregulated from baseline (respiratory rate < 25 bpm, peak airway pressure < 25 cm H2O, tidal volume < 6 mL/kg, PaCO2 < 40 mmHg) until PaCO2 increased by 5 − 10 mmHg. The pressure muscle index (PMI) and airway pressure swing during occlusion (ΔPOCC) were used to monitor respiratory effort, and they were measured before and after enforcement of the regulations.
Results
Ten patients with COVID-19 who had undergone ECMO were enrolled in this prospective study. When the PaCO2 increased from 36 (36 − 37) to 42 (41–43) mmHg (p = 0.0020), there was a significant increase in ΔPOCC [from 5.6 (4.7–8.0) to 11.1 (8.5–13.1) cm H2O, p = 0.0020] and PMI [from 3.0 ± 1.4 to 6.5 ± 2.1 cm H2O, p < 0.0001]. Meanwhile, increased inspiratory effort determined by elevated PaCO2 levels led to enhancement of tidal volume from 4.1 ± 1.2 mL/kg to 5.3 ± 1.5 mL/kg (p = 0.0003) and respiratory rate from 13 ± 2 to 15 ± 2 bpm (p = 0.0266). In addition, the increase in PaCO2 was linearly correlated with changes in ΔPOCC and PMI (R2 = 0.7293, p = 0.0003 and R2 = 0.4105, p = 0.0460, respectively).
Conclusions
In patients with COVID-19 undergoing PSV with ECMO, an increase of PaCO2 could increase the inspiratory effort.
Similar content being viewed by others
Background
Excessive respiratory effort may cause self-inflicted lung injury (SILI) and inspiratory muscle injuries [1,2,3], stimulate desynchronization between the patient and ventilator [4], and worsen the perfusion of extrapulmonary organs [5]. Appropriate respiratory drive and effort should be maintained during the treatment of patients with respiratory failure [6]. In contrast, respiratory drive and effort are commonly increased in patients with COVID-19 pneumonia [7], and this phenomenon may persist in critically ill patients with COVID-19, even after receiving venovenous extracorporeal membrane oxygenation (vv-ECMO) support, owing to low pulmonary compliance and a high systemic inflammatory state [8].
To reduce respiratory effort and drive, physicians often administer high doses of sedative drugs, analgesics, and muscle relaxants. The prolonged use of high doses of these drugs can cause loss of the spontaneous cough reflex, which in turn impairs sputum drainage and eventually worsens pulmonary consolidation and lung infections.
As the partial pressure of carbon dioxide in arterial blood (PaCO2) could affect the respiratory drive from the respiratory center [1], it has been shown that altering different levels of extracorporeal carbon dioxide removal in patients undergoing ECMO recovering from acute respiratory distress syndrome (ARDS) could alter respiratory drive [9]. We hope to explore the effect of PaCO2 level on respiratory effort in patients with COVID-19 undergoing ECMO.
Materials and methods
The study was performed based on the Declaration of Helsinki. All experiments were performed in accordance with relevant guidelines and regulations. The ethics committee of Peking Union Medical College Hospital approved this study(Ethics certificate number:K23C1385). Written informed consent was provided from the patients and from the next of kin of all enrolled patients.
Patient enrollment
The study was conducted in the intensive care unit of Peking Union Medical College Hospital in China. Patients with COVID-19 who had undergone ECMO and pressure support ventilation (PSV) via tracheal intubation between December 2022 and March 2023 were considered eligible for inclusion.
Drainage blood was drained using a 21 Fr cannula and return blood was drained using a 17 Fr cannula to achieve a blood flow of up to 5 L/min. ECMO blood flow was typically 3.0-3.5 L/min, while sweep gas flow (GF) was 3–9 L/min to maintain arterial oxygenation and normocapnia.
On admission, we recorded data on age, sex, predicted body weight, Sequential Organ Failure Assessment (SOFA) score, static respiratory system compliance, arterial blood gas analysis, ventilation analyses, ventilation and ECMO settings (blood flow, GF), and days on ECMO.
Measurements
Measurement of respiratory effort: 1) Pressure muscle index(PMI): Using the airway occlusion method, we put forward a simple estimate of the pressure developed by the inspiratory muscles at end inspiration. During the pressure support mode, the inspiratory hold button was pressed and a physician performed an end-inspiratory occlusion maneuver. After a certain period, the patient completely stopped inspiratory effort. The difference between the end-inspiratory obstructive plateau pressure and pre-obstructive airway pressure (Paw) was used to estimate the patient’s inspiratory effort and referred to as PMI [10, 11](Figure S1-A). 2) Airway pressure swing during occlusion (ΔPOCC): ΔPOCC is defined as the swing in the Paw generated by the force of the respiratory muscle under assisted ventilation when the airway is temporarily blocked [3]. The expiratory airway occlusion of the ventilator was performed at random intervals during each recording. Each occlusion persisted for a single breath, verified by the Paw recovery to normal. The maximum deviation of Paw from positive end-expiratory pressure (PEEP) during each occlusion was documented as ΔPOCC (Figure S1-B).
All patients were receiving mechanical pressure support ventilation (SV800 Ventilator, Mindray, Shenzhen, China) and monitoring of end tidal carbon dioxide (etCO2) (CAPNOSTAT M2501A CO2 Sensor, Philips, Netherlands).
Study protocol
A stable environment was maintained during the study to avoid stress and abrupt stimulation.
Before the start of the study, sedative drugs were titrated to Richmond agitation sedation scale values of − 3 to − 2, an assisted breathing mode trial was conducted, and support pressure level were adjusted to achieve tidal volume < 6 mL/kg. The ECMO GF was adjusted to achieve stable baseline conditions, defined as PaCO2 < 40 mmHg, respiratory rate < 25 bpm, and peak airway pressure < 25 cm H2O. PEEP, fraction of inspired oxygen, PSV, ECMO blood flow, and dose of norepinephrine, sedatives, and analgesics remained unchanged throughout the study.
The study protocol was initiated when the baseline parameters were stable. The baseline parameters, including ventilation settings, arterial and arterial blood gas analysis, hemodynamics, and indicators of respiratory effort were measured in the baseline phase. Then, the ECMO GF was modified to 50% of the baseline, and etCO2 values were monitored. ECMO GF was adjusted at 5-min intervals (increasing or decreasing by 0.5 L/min each time) until etCO2 stabilized at a level 5–10 mmHg higher than the baseline. After 20 min, the parameters were measured for the second time in the high-CO2 phase (Fig. 1).
In this study, the primary endpoint parameters were PMI and ΔPOCC, and the secondary endpoint parameters were respiratory parameters such as respiratory rate and tidal volume.
The study was stopped if the heart rate (HR) was > 140 bpm and/or respiratory rate was > 40 bpm and/or systolic blood pressure > 180 mmHg and/or patients experienced anxiety or diaphoresis.
Statistical analysis
Descriptive analysis was performed. All data are expressed as mean ± standard deviation or the median (25–75%, interquartile range). The Shapiro-Wilk test was used to evaluate normality. Variables were compared between the baseline and high-CO2 phase using the Student’s paired t-test or Wilcoxon matched-pairs signed-rank test. Linear correlations were analyzed using the Pearson’s test. In our pre-experiment, we found that the change in PaCO2 doubled ΔPOCC and PMI. The study was designed with 80% power to detect the minimum difference between the two phases, with a two-tailed alpha of 0.05. The calculated sample size was 9. Furthermore, the sample size was similar to that of previous studies [9, 12]. All comparisons were two-tailed, and a p < 0.05, was required to exclude the null hypothesis. SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis.
Results
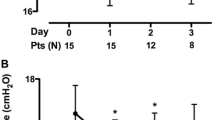
Ten patients with COVID-19 who had undergone ECMO were enrolled between December 2022 and March 2023. All patients successfully completed the study protocol. Nine patients received vv-ECMO with internal jugular-femoral vein access, while the drainage cannula was femoral venous cannula and the return one was right internal jugular venous catheter. One patient had a double-lumen cannula in the neck. Each patient had two phases of measurement data (baseline and high-CO2 phase). Demographic and basic hemodynamic parameters are presented in Table 1. All patients were men and with a mean age of 75 ± 9 years. On admission, the SOFA score was 12 ± 2, the Mechanical Ventilation(MV) time was 22 ± 15 days, and the mean ECMO time was 12 ± 2 days.
In order to increase the level of PaCO2 by 5–10 mmHg under PSV, the ECMO GF was decreased from baseline 5.8 (5.0–6.0) L/min to 2.9 (2.5–3.0) L/min in the high-CO2 phase (p = 0.0020). Arterial blood gas analysis showed that the patient’s PaCO2 increased from 36 (36–37) mmHg at baseline to 42 (41–43) mmHg in the high-CO2 phase (p = 0.0020) (Table 2).
After PaCO2 was increased by 5– 10 mmHg, there was a significant increase in ΔPOCC [from 5.6 (4.7–8.0) to 11.1 (8.5–13.1) cm H2O, p = 0.0020] and PMI [from 3.0 ± 1.4 to 6.5 ± 2.1 cm H2O, p < 0.0001] (Table 2; Fig. 2). Meanwhile, increased inspiratory effort determined by elevated PaCO2 levels led to enhancement of tidal volume from 4.1 ± 1.2 mL/kg to 5.3 ± 1.5 mL/kg (p = 0.0003) and respiratory rate from 13 ± 2 bpm to 15 ± 2 bpm (p = 0.0266) (Table 2).
In addition, the increase in PaCO2 was linearly correlated with changes in ΔPOCC and PMI (R2 = 0.7293, p = 0.0003 and R2 = 0.4105, p = 0.0460, respectively) (Fig. 3).
Correlation between the difference in PaCO2 and respiratory effort parameters. ΔPOCC, airway pressure swing during occlusion; PMI, pressure muscle index; ΔPaCO2, the value of PaCO2 in the High PaCO2 phase minus the base PaCO2 value; ΔΔPOCC, the value of ΔPOCC in the High PaCO2 phase minus the base ΔPOCC value; ΔPMI, the value of PMI in the High PaCO2 phase minus the base PMI value
However, the HR of the high-CO2 phase was higher than that of the baseline phase (89 ± 14 vs. 82 ± 14 bpm, p = 0.0078), meanwhile, with the same norepinephrine dose, there was no statistically significant difference in mean arterial pressure between the two phases.
Discussion
Herein, we analyzed the effect of PaCO2 on the respiratory drive in patients with COVID-19 who had undergone PSV with ECMO. Higher PaCO2 levels were associated with a greater respiratory drive.
Previously, it was shown that in removal of CO2 by ECMO could induce apnea in healthy and injured animal models [13]. Moreover, two studies showed similar results in patients on vv-ECMO. Marcolin et al. showed that in spontaneously breathing patients with acute respiratory failure, increased ECMO GF critically affected minute ventilation [14]; Moreover, Karagiannidis et al. showed an increase in diaphragm electrical activity (Edi) due to reduction in ECMO GF [15]. Mauri et al. showed that reducing CO2 removal by ECMO increased the first 100 min of inspiration against an occluded airway (P0.1) and ΔPOCC in patients who had undergone ECMO and recovering from ARDS through PSV and neurally adjusted ventilatory assist [9]. At the same time, the work of breathing, tidal volume, minute ventilation, and airway pressure also increased with the reduction in CO2 removal by ECMO. A recent study on the acute exacerbation of chronic obstructive pulmonary disease showed that the respiratory drive (assessed by Edi) increased in the unsuccessful and successful weaning phases during stepwise weaning from venovenous extracorporeal CO2 removal [16].
We observed the effect of PaCO2 on the respiratory effort of patients with COVID-19 who had undergone PSV with ECMO. Compared with the target value of PaCO2 at 35–40 mmHg, a higher PaCO2 (> 40 mmHg) was accompanied by a stronger respiratory effort.
Appropriate PaCO2 target
Based on our results, in patients with COVID-19 undergoing PSV with ECMO, an increase in PaCO2 level causes an enhanced respiratory effort. Thus, excessive respiratory effort may be able to be reduced in these patients by decreasing PaCO2. The benefits may be as follows: (1) Reducing the patient’s high respiratory effort, thus reducing SILI caused by trans-pulmonary pressure exceeding protective limits. (2) Appropriate respiratory drive is beneficial for maintaining patients with COVID-19 on ECMO in a state of spontaneous breathing with adequate choking capacity, which could help improve sputum drainage, promote lung aeration, improve lung compliance, and accelerate the improvement of COVID-19 pneumonia.
A linear correlation was also observed between the increase in PaCO2 and the elevation of ΔPOCC (R2 = 0.7293, p = 0.0003). The same phenomenon was also observed between PaCO2 and PMI (R2 = 0.4105, p = 0.0460). This may also confirm the role of PaCO2 levels in the regulation of respiratory effort in patients with COVID-19 on ECMO, providing a method for titrating the respiratory effort in these patients.
However, proper respiratory drive is necessary to maintain pulmonary aeration. In patients on long-term ECMO support, maintaining appropriate lung aeration can promote lung opening and decrease disuse myopathy [17]. Therefore, very low paCO2 level may not be necessary, which may cause acid-base disturbances and other pathophysiological conditions.
Timing of spontaneous breathing
In the early stages of severe ARDS, respiratory drive is often too strong, accompanied by excessive respiratory mechanical power. Therefore, spontaneous breathing in patients with severe ARDS undergoing ECMO was considered dangerous if it was used too early [18]. Furthermore, the general clinical practice suggests that attempts to perform spontaneous breathing in the early stages of severe ARDS are often impossible. A similar phenomenon was observed in this study. In the early days of vv-ECMO support, the patient’s spontaneous respiratory effort was often so strong that muscle relaxants along with analgesics and sedatives were required to control it. Therefore, patients in our study were on MV for 22 ± 15 days and ECMO support for 15 ± 16 days at the time of enrolment.
Based on our results, lower respiratory effort by increasing CO2 clearance in patients with COVID-19 on ECMO may also be appropriate in the early stages of the disease.
Therefore, whether lower PaCO2 is beneficial for perform spontaneous breathing earlier deserves further investigation.
Limitations
There were a few limitations to this study: (1) Ten patients were included in the study, similar to previous studies [9, 19]. However, their sample size was relatively small, which may have increased the occurrence of type II errors. (2) The PaCO2 alteration lasted only 20 min before the second measurement was taken but this appeared to be sufficient to obtain stable changes in respiratory patterns and circulatory alterations in previous studies [5, 9], thus making it unnecessary to continue the study for a longer period. (3) The enrolled patients were no longer in the early stages of COVID-19 pneumonia, as they were maintained in a spontaneous breathing state. Therefore, our results provide limited guidance for patients in the early stages of COVID-19. (4) A recent study showed that a significant relative decrease in PaCO2 within the first 24 h after ECMO initiation is associated with an increased incidence of neurological complications [20]. Unfortunately, data on cerebral perfusion did not be recorded and there were also no relevant neurological complications in the enrolled patients after our study. However, the results of that study suggested that a rapid drop in CO2 of more than 50% was dangerous, and a drop of less than 30% did not suggest harm. the CO2 drop in our study was relatively small, with a mean drop of 16%.
Conclusions
In patients with COVID-19 undergoing PSV with ECMO, an increase of PaCO2 could increase the inspiratory effort.
Data Availability
The data generated and analyzed during this study are not publicly available due to the protection for the patients’ privacy but are available from the corresponding author on reasonable request.
Abbreviations
- ECMO:
-
Extracorporeal membrane oxygenation
- Edi:
-
Diaphragm electrical activity
- etCO2 :
-
End tidal carbon dioxide
- FIO2 :
-
The fraction of inspired oxygen
- GF:
-
Gas flow
- HR:
-
Heart rate
- NAVA:
-
Neurally adjusted ventilatory assist
- PaCO2 :
-
The partial pressure of carbon dioxide in arterial blood
- Paw:
-
Airway pressure
- PEEP:
-
Positive end expiratory pressure
- PSV:
-
Pressure support ventilation
- PMI:
-
Pressure muscle index
- ΔPOCC:
-
Airway pressure swing during occlusion
- P0.1 :
-
The first 100ms of inspiration against an occluded airway
- ScvO2 :
-
Superior vena cava oxygen saturation
- SILI:
-
Self-inflicted lung injury
- SOFA:
-
Sequential organ failure assessment
- vv-ECMO:
-
Venovenous ECMO
References
Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically Ill patients. Pathophysiology and clinical implications. Am J Respir Crit Care Med. 2020;201(1):20–32.
Jonkman AH, de Vries HJ, Heunks LMA. Physiology of the respiratory drive in ICU patients: implications for diagnosis and treatment. Crit Care. 2020;24(1):104.
Bertoni M, Spadaro S, Goligher EC. Monitoring patient respiratory effort during mechanical ventilation: lung and diaphragm-protective ventilation. Crit Care. 2020;24(1):106.
Goligher EC, Ferguson ND, Brochard LJ. Clinical challenges in mechanical ventilation. Lancet. 2016;387(10030):1856–66.
Zhou Y, Chi Y, He H, Cui N, Wang X, Long Y. High respiratory effort decreases splanchnic and peripheral perfusion in patients with Respiratory Failure during mechanical ventilation. J Crit Care. 2023;75:154263.
Dianti J, Fard S, Wong J, Chan TCY, Del Sorbo L, Fan E, Amato MBP, Granton J, Burry L, Reid WD, et al. Strategies for lung- and diaphragm-protective ventilation in acute hypoxemic Respiratory Failure: a physiological trial. Crit Care. 2022;26(1):259.
Gattinoni L, Gattarello S, Steinberg I, Busana M, Palermo P, Lazzari S, Romitti F, Quintel M, Meissner K, Marini JJ et al. COVID-19 pneumonia: pathophysiology and management. Eur Respir Rev 2021, 30(162).
Li Bassi G, Suen JY, Dalton HJ, White N, Shrapnel S, Fanning JP, Liquet B, Hinton S, Vuorinen A, Booth G, et al. An appraisal of respiratory system compliance in mechanically ventilated covid-19 patients. Crit Care. 2021;25(1):199.
Mauri T, Grasselli G, Suriano G, Eronia N, Spadaro S, Turrini C, Patroniti N, Bellani G, Pesenti A. Control of respiratory drive and effort in extracorporeal membrane oxygenation patients recovering from severe Acute Respiratory Distress Syndrome. Anesthesiology. 2016;125(1):159–67.
Kyogoku M, Shimatani T, Hotz JC, Newth CJL, Bellani G, Takeuchi M, Khemani RG. Direction and magnitude of change in Plateau from peak pressure during Inspiratory holds can identify the degree of spontaneous effort and Elastic workload in ventilated patients. Crit Care Med. 2021;49(3):517–26.
Zhou Y, He H, Cui N, Wang H, Zhou X, Long Y. Acute hyperventilation increases oxygen consumption and decreases peripheral tissue perfusion in critically ill patients. J Crit Care. 2021;66:148–53.
Mauri T, Bellani G, Grasselli G, Confalonieri A, Rona R, Patroniti N, Pesenti A. Patient-ventilator interaction in ARDS patients with extremely low compliance undergoing ECMO: a novel approach based on diaphragm electrical activity. Intensive Care Med. 2013;39(2):282–91.
Langer T, Vecchi V, Belenkiy SM, Cannon JW, Chung KK, Cancio LC, Gattinoni L, Batchinsky AI. Extracorporeal gas exchange and spontaneous breathing for the treatment of acute respiratory distress syndrome: an alternative to mechanical ventilation?*. Crit Care Med. 2014;42(3):e211–220.
Marcolin R, Mascheroni D, Pesenti A, Bombino M, Gattinoni L. Ventilatory impact of partial extracorporeal CO2 removal (PECOR) in ARF patients. ASAIO Trans. 1986;32(1):508–10.
Karagiannidis C, Lubnow M, Philipp A, Riegger GA, Schmid C, Pfeifer M, Mueller T. Autoregulation of ventilation with neurally adjusted ventilatory assist on extracorporeal lung support. Intensive Care Med. 2010;36(12):2038–44.
Karagiannidis C, Strassmann S, Schwarz S, Merten M, Fan E, Beck J, Sinderby C, Windisch W. Control of respiratory drive by extracorporeal CO(2) removal in acute exacerbation of COPD breathing on non-invasive NAVA. Crit Care. 2019;23(1):135.
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–35.
Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fu**o Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med. 2012;40(5):1578–85.
Del Sorbo L, Goffi A, Tomlinson G, Pettenuzzo T, Facchin F, Vendramin A, Goligher EC, Cypel M, Slutsky AS, Keshavjee S, et al. Effect of driving pressure change during extracorporeal membrane oxygenation in adults with Acute Respiratory Distress Syndrome: a randomized crossover physiologic study. Crit Care Med. 2020;48(12):1771–8.
Cavayas YA, Munshi L, Del Sorbo L, Fan E. The early change in Pa(CO(2)) after extracorporeal membrane oxygenation initiation is Associated with neurological Complications. Am J Respir Crit Care Med. 2020;201(12):1525–35.
Acknowledgements
Not applicable.
Funding
This work was supported by the National High-Level Hospital Clinical Research Funding(2022-PUMCH-A-216), Central University Education and Teaching Reform Special Funding of Peking Union Medical College in 2023(2023zlgl022), the National High-Level Hospital Clinical Research Funding (2022-PUMCH-B-115).
Author information
Authors and Affiliations
Contributions
Yuankai Zhou contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting of the article, and final approval of the version to be published. **nchen Wang helped with data measurement and collection. Wei Du, Huaiwu He assisted in patient enrollment and quality control of data measurements; Na Cui contributed to the design of the study and helped to the discussion section, **aoting Wang helped the authors with the methodology of Doppler ultrasonography and provided valuable comments on the discussion section. Yun Long supervised the entire research program and is the corresponding author of this article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed based on the Declaration of Helsinki. All experiments were performed in accordance with relevant guidelines and regulations. The ethics committee of Peking Union Medical College Hospital approved this study (Ethics certificate number: K23C1385). Written informed consent was provided from the patients and from the next of kin of all enrolled patients.
Consent for publication
Not applicable.
Competing interests
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

12871_2023_2382_MOESM1_ESM.png
Supplementary Fig. S1: Graphical representation of PMI and ΔPOCC waveform. (A) PMI = the difference between end-inspiratory obstructive plateau pressure and pre-obstructive airway pressure (Paw). (B) ΔPOCC = the maximum deviation of Paw from PEEP during each expiratory airway occlusion
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Wang, X., Du, W. et al. The level of partial pressure of carbon dioxide affects respiratory effort in COVID-19 patients undergoing pressure support ventilation with extracorporeal membrane oxygenation. BMC Anesthesiol 24, 23 (2024). https://doi.org/10.1186/s12871-023-02382-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-023-02382-9