Abstract
Background
The Fructus Ligustri Lucidi, the fruit of Ligustrum lucidum, contains a variety of bioactive compounds, such as flavonoids, triterpenoids, and secoiridoids. The proportions of these compounds vary greatly during the different fruit development periods of Fructus Ligustri Lucidi. However, a clear understanding of how the proportions of the compounds and their regulatory biosynthetic mechanisms change across the different fruit development periods of Fructus Ligustri Lucidi is still lacking.
Results
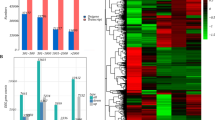
In this study, metabolite profiling and transcriptome analysis of six fruit development periods (45 DAF, 75 DAF, 112 DAF, 135 DAF, 170 DAF, and 195 DAF) were performed. Seventy compounds were tentatively identified, of which secoiridoids were the most abundant. Eleven identified compounds were quantified by high performance liquid chromatography. A total of 103,058 unigenes were obtained from six periods of Fructus Ligustri Lucidi. Furthermore, candidate genes involved in triterpenoids, phenylethanols, and oleoside-type secoiridoid biosynthesis were identified and analyzed. The in vitro enzyme activities of nine glycosyltransferases involved in salidroside biosynthesis revealed that they can catalyze trysol and hydroxytyrosol to salidroside and hydroxylsalidroside.
Conclusions
These results provide valuable information to clarify the profile and molecular regulatory mechanisms of metabolite biosynthesis, and also in optimizing the harvest time of this fruit.
Similar content being viewed by others
Background
Fructus Ligustri Lucidi (FLL) is the fruit of Ligustrum lucidum, which belongs to the Oleaceae family. Ligustrum lucidum is widely distributed in China, South Korea, India, and Australia and is often used for environmental greening. Its berry fruits, which are used as phytomedicine, are generally ovate, elliptic, or kidney-shaped, with a dark purple or grey-black color; they are usually harvested in winter (Fig. S1). In China, dried mature FLL is named Nvzhenzi, and it is used in traditional Chinese medicine (TCM) [1]. Modern chemical research has shown that triterpenes, phenylethanoid glycosides, secoiridoids, and flavonoids are the main secondary metabolites of FLL. These metabolites have a wide range of pharmacological effects, and are often used in clinical applications [2, 1]. Because of this, FLL is the main source of oleanolic acid in the market [2]. In our study, the ion intensity of oleanolic acid was stable across the different periods. Oleanolic acid biosynthesis has been demonstrated due to its various pharmacological effects [20]. Oleanolic acid can be generated from 2,3-oxidosqualene, which is generated from FPS by SQS and SQE. Then, 2,3-oxidosqualene is processed by BAS and CYP716A to produce oleanolic acid. The genes related to the MEP and MVA pathways were tended to be expressed in the 45 DAF and 75 DAF period. A large number of precursors substance of terpenoids may have been accumulated in these two sampling times. FPPS was highly expressed from 45 DAF to 135 DAF, guiding the compounds flow to triterpenes. These downstream genes, such as SQE, SQS, BAS, and CYP716A, were expressed in all the stages, and may be the reason for the stable oleanolic acid content, which was consistent with previous reports [21].
Secoiridoids, such as those found in FLL and olives, are abundant in Oleaceae and are widely used in food, lumber, cosmetics, edible olive oil, and medicine [22]. Oleuropein, the most abundant compound in olive oil, was the first oleoside-type secoiridoids reported [10]. In FLL, elevated levels of oleuropein were observed during the immature fruit periods, specifically 45 DAF, 75 DAF, and 112 DAF, but decreased as the fruit underwent physiological development. These observations align with what has been observed in olives. The extent to which oleuropein degradation occurs may be influenced by the activity of β-glucosidase [4]. β-glucosidase activity may have been higher at 135 DAF and 170 DAF than in the immature fruit stages, so the oleuropein content in these two stages was lower. However, the lowest oleuropein content was observed in dry FLL. It is possible that the transition from fresh fruit to dry FLL may affect the stability of this compound. Oleuropein was also enriched in olive oil that was extra-virgin cold-pressed rather than extracted, probably because it is heat-unstable. Due to their similar structures, ligstroside was also likely to undergo degradation by β-glucosidase, resulting a significant accumulation of its content at 45 DAF, 75 DAF, and 112 DAF, followed by a substantial decrease in the subsequent 135 DAF, 170 DAF and 195 DAF. In addition, specnuezhenide and nuezhenoside G13 both share a common β-glucose structure and are susceptible to the activity of β-glucosidase. This enzymatic action may lead to a reduction in their contents at 170 DAF and 195 DAF. In contrast, these two compounds exhibited higher levels of enrichment in dry FLL (fully ripened and dried fruits) compared to fresh fruits at 170 DAF and 195 DAF. It is possible that the activity of β-glucosidase was inhibited during the drying process, resulting in the cessation of the conversion of these two products. Additionally, the activity of catalytic enzymes inside the fruit continued for a certain period of time after harvesting, which may have led to an increase in the levels of these two compounds in dry FLL. Thus, the changes in secoiridoids, in addition to environmental factors, are closely related to their biosynthesis.
The biosynthetic route for the formation of oleoside-type secoiridoids has only been partially reported [23]. Based on their structures, it seems that spenuezhenide, oleuropein, nuezhenoside G13, ligstroside, and neonuzhenide were produced from OME-glu esterized with tyrosol, hydroxytyrosol, or their glucosides. OME-glu is a glycosylated product of OME, which is known to mediate oleoside biosynthesis. Glycosylation and esterification have not yet been demonstrated and characterized [4, 11]. The quantitative analysis of OME and OME-glu showed that there was almost no OME-glu in dry FLL, but a small amount of OME remained. When the content of oleosides such as spenuezhenide and G13 were lower during the six periods, OME-glu also had a lower content, while OME had a higher content (Fig. 1). This variation in the contents of these compounds suggested that the conversion of OME to OME-glu was slow in the later periods (135 DAF to 195 DAF), while the consumption of OME-glu was rapid. During these stages, oleoside content was stable but OME-glu content increased slightly, suggesting that the GT activity of OME on OME-glu decreased gradually in the later harvesting times. To explore the specific GT of OME, we isolated the total FLL protein caused it to react OME. However, the expected chromatographic peak of OME-glu did not appear (Fig. S12). Compared with the deactivation protein, the peak area of OME obviously decreased in the total protein reaction. This may be because the total protein extract was more complex and contained other small-molecule compounds. OME-glu might have reacted rapidly and therefore would not have been detected. According to studies on plant acyltransferases, it is highly probable that enzymes with esterification activity belong to the serine carboxypeptidase acyltransferase (SPCL) family, which utilizes 1-O-β-glucose esters as acyl donors [24]. The structure of OME-glu can be recognized as a 1-O-β-glucose ester that react with tyrosol, although this has not yet been demonstrated. The esterification activity may also be performed using BAHD, which is named after the first four biochemically characterized ATs, namely benzylalcohol O-acetyltransferase, anthocyanin O-hydroxycinnamoyltransferase, anthranilate N-hydroxycinnamoyl/benzoyltransferase, and deacetylvindoline 4-O-acetyltransferase [24]. Hence, the biological function and biocatalytic potential of esterification and glycosylation warrant further study. These two unknown catalytic steps can potentially be elucidated based on the analysis of the complete genome. In addition, oleoside-type secoiridoids are predominantly found in the Oleaceae family, including plants such as Osmanthus fragrans, olive, and FLL. These plants belong to different genera and exhibit distinct geographical distribution characteristics, yet they have the capability to produce compounds with similar structures. The co-evolution of these plants within the secoiridoids biosynthetic pathway could potentially explain the occurrence of this phenomenon.
Oleoside-type secoiridoids, such as oleuropein and ligstroside, contain phenolic moieties such as tyrosol, hydroxytyrosol, and their C8-glycosylation salidroside and hydroxysalidroside. The production of these compounds is important for the biosynthesis of oleoside-type secoiridoids [15]. Tyrosine can react with TyDCs, MAO, and AAS to produce 4-HPAA. Because of the high amino acid sequence identity between TyDC and AAS, they cannot be separated using primary sequence analysis, so them were grouped for analysis [25]. There were two expression trends of TyDC/AAS in FLL; therefore, the functions of the sequences require further study. The specific glycosylation step of tyrosol has been explored in R. rosea. Genes homologous to RrT4GT and RrT8GT were cloned and investigated for their potential roles in the glycosylation of tyrosol and hydroxytyrosol (Fig. 5). The maximum likelihood (ML) tree of these homologs was consistent with their protein similarity (Fig. S7D). However, the T4GT homologs, such as Cluster-13715.51696, Cluster-13715.41413, and Cluster-13715.7146, did not have T4GT activities. In contrast, the T8GTs 35,728, 18,726, and 48,377 converted tyrosol to salidroside. 35,728, 18,726, and 48,377 were found to be highly expressed in our RNA-seq data (Fig. S7E). Among these, Cluster-13715.18726 has excellent activity, and can completely catalyze tyrosol and hydroxytyrosol. It is unfortunate that the proteins with esterification and glycosylation in the biosynthesis of oleoside-type secoiridoids are still unknown. In order to investigate the biosynthesis of oleosides and characterize the specific enzymes involved, a better understanding of the transition of the compounds between oleosides is essential. This knowledge will enable the reconstitution and optimization of oleoside metabolic pathways, and lays the foundation for the comprehensive development and utilization of FLL to utilize its health-promoting properties. The results of this study provide a basis for reasonable selection of harvest time of FLL, and also provide a foundation for a comprehensive use and development of FLL resources.
Conclusions
In this study, transcriptomic and metabolomic analyses revealed the rules that describe the dynamic changes in the compounds comprising FLL across development periods. Metabolomic data identified 70 compounds across development periods, most of which were secoiridoids. Eleven other compounds were also quantified, such as tyrosol, salidroside, specnuezhenide, oleuropein, G13. The marker compound specnuezhenide was significantly upregulated at 112 DAF, and G13 was enriched at 135 DAF, the coloring period. Oleuropein was abundant at 45 DAF when FLL was in the young fruit stage. Using transcriptome data, we analyzed the biosynthetic pathways of the main components of FLL. In secoiridoid biosynthesis, gene expression was similar between SXS/OMES and ISY, suggesting that they may be co-expressed that the upregulation of these genes may be beneficial for oleuropein biosynthesis. The in vitro enzyme activity of GTs, similar to T4GT and T8GT, showed their catalytic ability to convert tyrosol and hydroxytyrosol to salidroside and hydroxysalidroside, respectively. Therefore, this study provides valuable information about the harvest period of FLL according to how the compounds accumulate. It also lays foundation for the molecular research on the improvement of FLL quality.
Materials and methods
Plant materials and standards
The samples in the present study were harvested from plants at Nan**g University of Chinese Medicine (Nan**g, China), which were identified by the corresponding author of this article (Professor Qinan Wu). Three fruit trees with good growing conditions at the same developmental period were selected, and sampling of fruits was carried out on August 16, 2021 (45 days after flowering, DAF), September,16 2021 (75 days after flowering), October 23, 2021 (112 days after flowering when the color of the exocarp began to change), November 16, 2021 (135 days after flowering), December 16, 2021 (170 days after flowering when the day of the Winter Solstice Festival in China is known to be the best time for harvesting), and January 16, 2022 (195 days after flowering) (Fig. S1). The six sampling times were named as 45 DAF, 75 DAF, 112 DAF, 135 DAF, 170 DAF, 195 DAF, respectively. The harvested samples were quickly frozen in liquid nitrogen and stored at -80℃. The dry FLL served as the standard traditional Chinese medicine which was used as control. Three biological replicates were used for each experiment.
Specnuezhenide, oleanolic acid, ursolic acid, salidroside, tyrosol, neonuezhenide, ligustroflavone, nuezhenoside G13, oleoside-11-methyl ester (OME), and oleuropein standards were purchased from Chengdu DeSiTe Biological Technology (Chengdu, China). Hydroxysalidroside and hydroxytyrosol ligstroside were purchased from Shanghai Yuanye Bio-Technology (Shanghai, China), and 7-β-1-D-glucopyranosyl-11-methyl oleoside (OME-glu) was purchased from MolPort (Riga, Latvia). HPLC-grade acetonitrile was purchased from Merck (Germany), and MS-grade formic acid was purchased from Sigma-Aldrich (Germany). All other chemicals and solvents used were of analytical grade. Ultrapure water was purchased from Watsons (Hongkong, China). The standards were all dissolved in methanol.
Sample preparation for LC-MS
The biological samples were freeze-dried using a vacuum freeze-dryer (Scientz-100 F). The freeze-dried samples were crushed using a mixer mill (MM 400, Retsch) with zirconia beads for 1.5 min at 30 Hz. Then, 0.3 g lyophilized powder was added to 10 mL 80% methanol solution and treated with ultrasound for 30 min three times. After centrifugation at 12,000 g for 10 min, the extracts were filtered through a 0.22-µm membrane filter before LC-MS/MS analysis. There were six biological repeats in each group.
The metabolite profiles were analyzed using a triple TOF 5600 + system (AB SCIEX, Foster City, CA, USA) with an ESI source coupled to a UPLC system (Shimadzu 30 A UHPLC system, Shimadzu, Japan). For UPLC analysis, a 2 µL sample was injected into an analytical reverse-phase column (XBridge C18 Column 150 mm × 4.6 mm, 3.5 μm). Separation was performed with 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The total running time was 45 min and the flow rate was 0.6 mL/min. The column compartment was maintained at 30 °C. The mobile phase had a gradient elution of 5 − 20% B (0–5 min), 20 − 35% B (5–21 min), 35 − 70% B (21–22 min), 70 − 97% B (22–38 min), 97% − 5% B (38–42 min) and 5% B (42–45 min). For TOF analysis, the mass spectrometer was operated in the positive and negative ESI modes with a duo-spray source, and the mass scan range was set at m/z 50-1500 for both TOF-MS and TOF-MS/MS scans. The detailed parameters used were as described previously [26].
Quantitative analysis
A Waters Acquity HPLC™ system (e2695; Waters Corp., MA, USA) equipped with a TUV detector system (2998; Waters Corp.) was used for the quantitative analysis of specnuezhenide, salidroside, nuezhenoside G13, hydroxysalidroside, oleuropein, ligstroside, ligustroflavone, OME, and OME-glu. Chromatography was performed on an XBridge C18 column (250 m × 4.6 mm, 5 μm). The mobile phase consisted of acetonitrile (A) and 0.15% formic acid in water (B). The HPLC elution conditions were optimized as follows: 0–20 min, 10 − 18% A; 20–30 min, 18 − 35% A; 30–52 min, 35 − 69% A; 52–60 min, 69% − 10% A; 60–65 min, 10% A. The flow rate was 1.0 mL/min, the column was maintained at 28 ℃, the injection volume was 10 µL, and the detection wavelength was set at 253 nm. Based on a previous method, an external calibration method was used for the quantitative analysis [9]. Linear calibration curves were constructed for specnuezhenide (y = 4778.2x + 5653.0), salidroside (y = 2415.1x + 1912.0), nuezhenoside G13 (y = 3649.0x + 3192.3), hydroxysalidroside (y = 738.22x − 234.77), oleuropein (y = 6978.6x + 5503.8), ligstroside (y = 5000.3x + 1726.9), ligustroflavone (y = 6629.5x − 220.73), OME (y = 6829.9x + 4991.3), and OME-Glu (y = 2276.5x + 8697.5). Good linear correlation and high sensitivity under these chromatographic conditions were confirmed using correlation coefficients (R > 0.999) (Table S1). The extracts used for LC-MS analysis were injected into an HPLC system for the quantitative analysis of the chemicals mentioned above. The data are expressed as mean values with standard deviation and were obtained from six biological replicates.
RNA extension and transcriptome analysis
Total RNA of fruit samples harvested at different times was extracted using the CTAB reagent (QIAGEN, Germany). RNA samples were separately sequenced using an Illumina NovaSeq 6000 platform (Illumina, USA) following the manufacturer’s recommendations, in which the mRNA was purified using poly-T oligo-attached magnetic beads. Library quality was evaluated using an Agilent 2100 bioanalyzer. After each library was qualified, the different libraries were pooled according to the effective concentration and the target amount of data from the machine and then sequenced using an Illumina NovaSeq 6000 system (Illumina). There were three biological repeats in RNA-Seq.
Assembly, annotation, and differential gene expression analysis
Raw data were cleaned by removing low-quality reads, adapter reads, and N-containing reads. Trinity software (v2.6.6) was used to assemble the clean reads. Annotation was performed using the following databases: Nr, Nt, Pfam, COG/KOG (Clusters of Orthologous Groups of proteins), Swiss-Prot, KEGG, and GO. Differential expression analyses of the different development periods were performed using the DESeq2 R package (v1.20.0). The resulting P-values were adjusted using Benjamini and Hochberg’s approach to control the false discovery rate. Padj < 0.05 and |log2(foldchange)| > 1 were set as thresholds for significantly differentially expressed genes. Based on the results of the analyses, GOseq (v1.10.0) and KOBAS (v2.0.12) software were used for GO function enrichment analysis and KEGG pathway enrichment analysis of the differential gene sets.
Quantitative real-time PCR analysis
The qRT-PCR analysis was performed using a QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific Inc., Waltham, MA, USA) and a ChamQ Universal SYBR qPCR kit (Vazyme Biotech, Nan**g, China). The amplification procedure and qRT-PCR reaction solution was performed as previously described [24], with three biological replicates. ACTIN was used as the internal reference gene. Finally, relative gene expression was calculated using the 2−∆∆Ct method. All primers used for the qRT-PCR analyses are listed in Table S2.
Gene identification
The candidate genes in the biosynthesis were firstly selected using their KEGG and gene annotations; then each gene was blasted in NCBI manually. Cr7DLH (AGX93062.1), CrLAMT (AGX93063.1), CrSLS (AAA33106.1), Oe7eDLH (ALV83449.1), OeOMES (QOW17548.1), OeSXS (QOW17550.1), and OeLAMT (AFS28695.1) were used to search for homologous genes in FLL. The T4GTs and T8GTs of FLL were homologous genes of RrT4GT (AUI41117.1) and RrT8GT (AUI41147.1), respectively. RrT4GT and RrT8GT were set as query sequences, and FLL proteins were set as subject sequences with an E-value of 10 − 5. Sequences whose weight coverage was > 0.99 and with a subject_evalue of zero were selected, then the sequence lengths were checked. Sequences with similar lengths and having the same conserved domain were reserved. These sequences were then used in an enzymatic activity assay. The maximum likelihood (ML) tree of the T4GTs and T8GTs used MEGA X software (Mega Software, Dortmund, Germany) with 1,000 bootstrap-based JTT + G + I amino acid substitution models.
Gene isolation, enzyme expression, and enzymatic activity assay
A HiScript III 1st Strand cDNA Synthesis Kit (+ gDNA wiper) (Vazyme Biotech) was used with 0.5 µg of total RNA to synthesize the first-strand cDNA, which was used as the template for PCR with a 2 × Taq Master Mix (Vazyme Biotech). Gene-specific primers were designed based on annotated results from the transcriptome database. The PCR products were separated using a GeneJETTM Gel Extraction Kit (Thermo Fisher Scientific), and the purified DNA fragments were cloned into a pET28a vector using a ClonExpress II One Step Cloning Kit (Vazyme Biotech). All clones were sequenced using Sanger sequencing (Tsingke, Bei**g, China). The recombinant vector was transformed into Escherichia coli BL21 cells (TIANGEN, Bei**g, China) using the heat shock treatment method at 42 ℃ for 45 s. Finally, transformants were plated on LB medium containing 100 ng/L kanamycin.
Single colonies were used to inoculate into LB medium (5 mL) containing 100 ng/L kanamycin. The culture (1 mL) was transferred to 100 mL of the same medium and continued to grow at 37 °C to reach an OD600 of approximately 1.0. Protein expression was induced by adding isopropyl β-d-thiogalactopyranoside at a final concentration of 1.0 mM. After incubation at 18 ℃ for 20 h, the cells were harvested at 5,000 g, 4 ℃ for 15 min and resuspended in 2 mL lysis buffer (15% v/v glycerinum, 10 mM Tris-HCl [pH 8.0], and 200 mM NaCl). The suspension was then transferred to an ultrasonic cell disruptor system and ultrasonically disrupted for 25 s each, with 35 s on ice between disruptions with three times. The mixture was then centrifuged (30 min, 4 °C, 12,000 g), and the supernatant was carefully collected as the crude protein.
The reaction system of enzyme activity in vitro is as follows: the crude protein (40 µL), 50 mM Tris-HCl (pH 7.5), 500 µM substrate, 2 mM UDPG. They were added to a 250 µL reaction system, and incubated at 30 ℃ in the dark on a shaker. The reaction was stopped by adding 250 µL of methanol. Next, all samples were centrifuged at 12,000 g for 10 min and, the supernatant was subjected to HPLC analysis.
The HPLC system used was the same as that for the quantitative analysis. Chromatography was performed on an XBridge C18 column (250 m × 4.6 mm, 5 μm). The mobile phase consisted of acetonitrile (A) and 0.1% formic acid in water (B). The HPLC elution conditions were optimized as follows: 0–8 min, 10- 20% A; 8–10 min, 20 − 40% A;10–13 min, 40 − 70% A; 13–16 min, 70 − 95%; 16–19 min, 95%; 19–21 min, 95% − 10%; 21–25 min, 10%. The flow rate was 0.7 mL/min, the column was maintained at 30 ℃, and the injection volume was set as 10 µL.
Data availability
The datasets generated and/or analyzed during the current study are available in the Sequence Read Archive (SRA repository), under the Accession number PRJNA874863 [https://www.ncbi.nlm.nih.gov/bioproject/PRJNA874863] at NCBI. The RNA-seq data has been submitted to NCBI SRA: PRJNA874863 (The data will be available after the study is published).
Abbreviations
- FLL:
-
Fructus Ligustri Lucidi
- TCM:
-
traditional Chinese medicine
- 7eDLH:
-
7-deoxy-loganic acid 7-epi-hydroxylase
- OMES:
-
oleoside methyl ester synthase
- SXS:
-
secoxyloganin synthase
- GT:
-
glycosyltransferase
- HPLC:
-
High Performance Liquid Chromatography
- MVA:
-
mevalonic acid
- MEP:
-
methylerythritol phosphate
- DMAPP:
-
dimethylallyl diphosphate
- AACT:
-
acelyl-CoA C-acetyltransferas
- HMGCS:
-
hydroxymethylglutaryl-CoA synthase
- HMGCR:
-
hydroxymethylglutaryl-CoA reductase
- MVK:
-
mevalonate kinase
- PMK:
-
phosphomevalonate kinase
- DXS:
-
1-deoxy-D-xylulose-5-phosphate synthase
- DXR:
-
1-deoxy-D-xylulose-5-phosphate reductase
- MCT:
-
2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase
- CMK:
-
4-diphosphocytidyl-2-C-methyl-D-erythritol kinase
- MDS:
-
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase
- HDS:
-
(E)-4-hydroxy-3-methylbut-2-enyl-diphosphate synthase
- HDR:
-
4-hydroxy-3-methylbut-2-en-1-yl diphosphate reductase
- FPPS:
-
farnesyl pyrophosphate synthase
- SQE:
-
squalene monooxygenase
- SQS:
-
squalene synthase
- GES:
-
geraniol synthase
- GPP:
-
geranyl diphosphate
- G10H:
-
geraniol 10-hydroxylase
- 10GHO:
-
10-hydroxygeraniol oxidoreductase
- ISY:
-
iridoid synthase
- IO:
-
iridoid oxidase
- 7DLGT:
-
7-deoxyloganetic acid glucosyltransferase
- 7DLH:
-
7-deoxyloganic acid 7-hydroxylase
- LAMT:
-
loganic acid O-methyltransferase
- SLS:
-
secologanin synthase
- TyDC:
-
Tyrosine/Dopa decarboxylase
- 4-HPAA:
-
4-hydroxyphenylacetaldehyde
- 3,4-DHPAA:
-
3,4 dihidroxyphenylacetaldehyde
- MAO:
-
monoamino oxidase
- PAR:
-
phenylacetaldehyde reductase
- T8GT:
-
UDP-glucose 8-O-glucosyltransferase
- AAS:
-
aromatic aldehyde synthase
- T4GT:
-
UDP-glucose 4-O-glucosyltransferase
- F3H:
-
flavanone 3-hydroxylase
- F3’H:
-
flavonoid 3′-hydroxylase
- DFR:
-
dihydroflavonol 4-reductase
- ANS:
-
anthocyanidin synthase
- UFGT:
-
UDP-glucose: flavonoid 3-O-glucosyltransferase
- SPCL:
-
serine carboxypeptidase acyltransferase
- DAF:
-
days after flowering
References
Chen B, Wang L, Li L, Zhu R, Liu H, Liu C, Ma R, Jia Q, Zhao D, Niu J, et al. Fructus Ligustri Lucidi in osteoporosis: a review of its Pharmacology, Phytochemistry, Pharmacokinetics and Safety. Molecules. 2017;22(9):1469.
Gao L, Li C, Wang Z, Liu X, You Y, Wei H, Guo T. Ligustri lucidi fructus as a traditional Chinese medicine: a review of its phytochemistry and pharmacology. NAT PROD RES. 2015;29(6):493–510.
Zhang Y, **ao F, Zhou Q, Diao T, Zhang M, Liu D, Wang Z, Huang T, Wu Y, Bai Y, et al. The potential protective effect of Iridoid glycosides isolated from Osmanthus fragrans seeds against the development of Immune Liver Injury in mice. FRONT PHARMACOL. 2021;12:760338.
Koudounas K, Thomopoulou M, Rigakou A, Angeli E, Melliou E, Magiatis P, Hatzopoulos P. Silencing of Oleuropein β-Glucosidase abolishes the Biosynthetic Capacity of secoiridoids in Olives. FRONT PLANT SCI 2021, 12.
Zheng T, Yang X, Wu D, **ng S, Bian F, Li W, Chi J, Bai X, Wu G, Chen X, et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3beta pathway. Br J Pharmacol. 2015;172(13):3284–301.
Zhu L, Liu Z, Ren Y, Wu X, Liu Y, Wang T, Li Y, Cong Y, Guo Y. Neuroprotective effects of salidroside on ageing hippocampal neurons and naturally ageing mice via the PI3K/Akt/TERT pathway. PHYTOTHER RES. 2021;35(10):5767–80.
Gousiadou C, Kokubun T, Martins J, Gotfredsen CH, Jensen SR. Iridoid glucosides in the endemic Picconia Azorica (Oleaceae). Phytochemistry. 2015;115:171–4.
Volk J, Sarafeddinov A, Unver T, Marx S, Tretzel J, Zotzel J, Warzecha H. Two novel methylesterases from Olea europaea contribute to the catabolism of oleoside-type secoiridoid esters. Planta. 2019;250(6):2083–97.
Chen J, Wen Y. Effects of three Processing methods on changes of Seven Chemical constituents in Ligustri Lucidi Fructus. MODCHIN MED. 2021;23(07):1260–5.
Alagna F, Geu-Flores F, Kries H, Panara F, Baldoni L, O’Connor SE, Osbourn A. Identification and characterization of the Iridoid synthase involved in Oleuropein Biosynthesis in Olive (Olea europaea) Fruits. J BIOL CHEM. 2016;291(11):5542–54.
Rodríguez López CE, Hong B, Paetz C, Nakamura Y, Koudounas K, Passeri V, Baldoni L, Alagna F, Calderini O, O’Connor SE. Two bi-functional cytochrome P450 CYP72 enzymes from olive (Olea europaea) catalyze the oxidative C-C bond cleavage in the biosynthesis of secoxy-iridoids - flavor and quality determinants in olive oil. NEW PHYTOL. 2021;229(4):2288–301.
Miettinen K, Dong L, Navrot N, Schneider T, Burlat V, Pollier J, Woittiez L, van der Krol S, Lugan R, Ilc T, et al. The seco-iridoid pathway from Catharanthus roseus. NAT COMMUN. 2014;5:3606.
Sherden NH, Lichman B, Caputi L, Zhao D, Kamileen MO, Buell CR, O’Connor SE. Identification of iridoid synthases from Nepeta species: Iridoid cyclization does not determine nepetalactone stereochemistry. Phytochemistry. 2018;145:48–56.
De Dugé T, Foureau E, Parage C, Lanoue A, Clastre M, Londono MA, Oudin A, Houillé B, Papon N, Besseau S et al. Characterization of a second secologanin synthase isoform producing both secologanin and secoxyloganin allows enhanced de novo assembly of a Catharanthus roseus transcriptome. BMC Genomics 2015, 16(1).
Mougiou N, Trikka F, Trantas E, Ververidis F, Makris A, Argiriou A, Vlachonasios KE. Expression of hydroxytyrosol and oleuropein biosynthetic genes are correlated with metabolite accumulation during fruit development in olive, Olea europaea, Cv. Koroneiki. PLANT PHYSIOL BIOCH. 2018;128:41–9.
Bai Y, Bi H, Zhuang Y, Liu C, Cai T, Liu X, Zhang X, Liu T, Ma Y. Production of salidroside in metabolically engineered Escherichia coli. SCI REP. 2014;4:6640.
W HL, Q Y, B B LJX. M S, Y C, F C, A D, L Z: Application of UHPLC-ESI-Q-TOF-MS to Identify Multiple Constituents in Processed Products of the Herbal Medicine Ligustri Lucidi Fructus. MOLECULES 2017, 22(5).
Romani A, Ieri F, Urciuoli S, Noce A, Marrone G, Nediani C, Bernini R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. NUTRIENTS 2019, 11(8).
Zhang X, **e L, Long J, **e Q, Zheng Y, Liu K, Li X. Salidroside: a review of its recent advances in synthetic pathways and pharmacological properties. CHEM BIOL INTERACT. 2021;339:109268.
Wang Y, Zhang H, Ri HC, An Z, Wang X, Zhou JN, Zheng D, Wu H, Wang P, Yang J, et al. Deletion and tandem duplications of biosynthetic genes drive the diversity of triterpenoids in Aralia elata. NAT COMMUN. 2022;13(1):2224.
Yang Y, Liu X, Tan S, Wu W. Determination of oleanolic acid and ursolic acid in Fructus Ligustri Lucidi at different Har-vest time by HPLC. JOURNAL JIANGXI UNIVERSITY TCM. 2018;30(06):78–80.
Obied HK, Prenzler PD, Ryan D, Servili M, Taticchi A, Esposto S, Robards K. Biosynthesis and biotransformations of phenol-conjugated oleosidic secoiridoids from Olea europaea L. NAT PROD REP. 2008;25(6):1167.
Gutierrez-Rosales F, Romero MP, Casanovas M, Motilva MJ, Mínguez-Mosquera MI. β-Glucosidase involvement in the formation and Transformation of Oleuropein during the growth and development of Olive fruits (Olea europaea L. Cv. Arbequina) grown under different Farming practices. J AGR FOOD CHEM. 2012;60(17):4348–58.
Wang L, Chen K, Zhang M, Ye M, Qiao X. Catalytic function, mechanism, and application of plant acyltransferases. CRIT REV BIOTECHNOL. 2022;42(1):125–44.
Torrens-Spence MP, Pluskal T, Carballo V, Weng J. Complete pathway elucidation and heterologous reconstitution of Rhodiola Salidroside Biosynthesis. MOL PLANT. 2018;11(1):205–17.
Zhou P, Yin M, Dai S, Bao K, Song C, Liu C, Wu Q. Multi-omics analysis of the bioactive constituents biosynthesis of glandular trichome in Perilla frutescens. BMC PLANT BIOL 2021, 21(1).
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of China (82204597). Ecological Planting and Quality Assurance Project of Authentic Medicinal Materials (2022). The Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_1759 and KYCX22_2031).
Author information
Authors and Affiliations
Contributions
P.Z., C.Q., and Q.W. designed the study. Z.J. collected the samples. J.D., P.Z., and S.D. performed the experiments. P.Z. and J.D. analyzed and interpreted the data. P.Z., C.Q., and Q.W. wrote, reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The authors confirmed that the experimental research on plants (either cultivated or wild), including the collection of plant material comply with relevant institutional, national, and international guidelines and legislation. The samples were collected from wild populations in non-protected areas and cultivated and preserved in the experimental base of Nan**g University of Chinese medicine; no permissions/licences are required. The voucher specimen is stored in the Herbarium of nan**g university of Chinese medicine, with the code 3G340456, Qinan Wu identified it.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, P., Dang, J., Jiang, Z. et al. Transcriptome and metabolome analysis revealed the dynamic change of bioactive compounds of Fructus Ligustri Lucidi. BMC Plant Biol 24, 489 (2024). https://doi.org/10.1186/s12870-024-05096-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05096-3