Abstract
Background
Dioecy, a sexual system of single-sexual (gynoecious/androecious) individuals, is rare in flowering plants. This rarity may be a result of the frequent transition from dioecy into systems with co-sexual individuals.
Results
In this study, co-sexual expression (monoecy and hermaphroditic development), previously thought to be polyploid-specific in Diospyros species, was identified in the diploid D. oleifeara historically. We characterized potential genetic mechanisms that underlie the dissolution of dioecy to monoecy and andro(gyno)monoecy, based on multiscale genome-wide investigations of 150 accessions of Diospyros oleifera. We found all co-sexual plants, including monoecious and andro(gyno)monoecious individuals, possessed the male determinant gene OGI, implying the presence of genetic factors controlling gynoecia development in genetically male D. oleifera. Importantly, discrepancies in the OGI/MeGI module were found in diploid monoecious D. oleifera compared with polyploid monoecious D. kaki, including no Kali insertion on the promoter of OGI, no different abundance of smRNAs targeting MeGI (a counterpart of OGI), and no different expression of MeGI between female and male floral buds. On the contrary, in both single- and co-sexual plants, female function was expressed in the presence of a genome-wide decrease in methylation levels, along with sexually distinct regulatory networks of smRNAs and their targets. Furthermore, a genome-wide association study (GWAS) identified a genomic region and a DUF247 gene cluster strongly associated with the monoecious phenotype and several regions that may contribute to andromonoecy.
Conclusions
Collectively, our findings demonstrate stable breakdown of the dioecious system in D. oleifera, presumably also a result of genomic features of the Y-linked region.
Similar content being viewed by others
Background
Dioecy—male and female reproductive organs in separate individuals—is found in only approximately 6% of angiosperm species, but in diverse plant lineages [1,2,3]. This low rate of dioecy may indicate an evolutionary dead-end scenario [4]; however, it has been proposed that the dissolution of dioecy, which leads to re-evolution of the co-sexual systems, occurs during sexual-system evolution [5]. Multiple empirical studies have yielded results consistent with this hypothesis [6, 7].
Sex expression in the genus Diospyros is diverse, and several key genetic components controlling sexual expression have been identified [8]. Diploid D. lotus is dioecious, exhibiting separate male and female plants; polyploid D. kaki encompasses gynoecious (female flower only), androecious (male flower only), monoecious (male and female flowers), androgynomonoecious (male, female, and hermaphroditic flowers), and andromonoecious (male and hermaphroditic flowers) individuals [9,10,11,12]. Similar sexual diversity was observed in diploid D. oleifera (Fig. 1; Figs. S1-3). In Diospyros, male flowers are typically observed in the form of a three-flower cyme, but female flowers are always solitary (Fig. 1). Dioecy is considered an ancestral form in Diospyros; therefore, it is reasonable to assume that the polygamous system in D. kaki and D. oleifera evolved from the dioecious system [13]. In diploid dioecious D. lotus, the small RNA (smRNA)-coding gene OGI determines the formation of male trees via repression of the feminising gene MeGI [8]. In contrast, an additional layer of regulation in the form of DNA methylation of the MeGI promoter may contribute to monoecy in hexaploid D. kaki [10].
Diverse sex expressions in D. oleifera. A male floral buds, B female floral buds, C hermaphroditic floral buds, and D female flower cyme in pseudo-monoecy. E Schematic representation of individual sexual types. Details of the sexual types are shown in Figs. S1-3. Gynoecy and androecy represent female and male individuals in a dioecious population, respectively. Monoecy represents individuals bearing both female and male flowers. Andromonoecy represents individuals bearing both male and hermaphroditic flowers. Androgynomonoecy represents an individual bearing female, male and hermaphroditic flowers. Pseudo-monoecy represents individuals bearing two- or three-flower cymes with a female (or occasionally hermaphroditic) flower in the middle and one or two abnormal small female flowers at the sides
Although several important molecular mechanisms underlying the sexuality determination of D. spp. have been identified, the fundamental molecular mechanisms that underlie the sexual diversity of some D. spp. (e.g., D. kaki and D. oleifera) are unclear [14]. In this study, we used a genetic approach to characterise a D. oleifera population. The diploidy of D. oleifera with all types of sex expressions was confirmed (Method S1; Text S1; Fig. S4). The genome of diploid D. oleifera is simpler than the genome of cultivated polyploid D. kaki, and a new D. oleifera genome was assembled at the chromosome scale (Text S2; Figs. S5-S17; Tables S1-S12) [15]. The development of D. oleifera floral buds is synchronous to the development of D. kaki. Therefore, the analysis of D. oleifera could be a novel addition for deeper understanding of sex diversity in Diospyros.
In mid-April 2019 and 2021, we surveyed the sex phenotype of D. oleifera obtained from a natural population in Guilin City, Guangxi Zhuang Autonomous Region, China (25''04′46.08–25''56′14.00 N; 110''17′57.65–111''03′23.98 E) (Fig. S18). Two hundred eight plants with various types of sex expressions were sampled in total (Table 1; Table S13). The genetic components of the OGI/MeGI system were examined in the D. oleifera population. Subsequently, comparative-methylome and transcriptome analyses were performed to identify the molecular mechanism responsible for sexual diversity. Moreover, a genome-wide association study (GWAS) was conducted to determine candidate genomic regions and genes that contribute to the sexual diversity.
Results
Characterisation of the male-specific region (the MSR) in D. oleifera
We analysed the presence of the MSR [16] and the OGI gene in 150 D. oleifera individuals (Table 1; Table S13). We found that 54 of 58 gynoecious individuals did not contain the MSR or OGI (OGI-negative, OGI−). In contrast, all 45 androecious individuals contained the MSR and OGI (OGI-positive, OGI+). All co-sexual plants (monoecious, androgynomonoecious, and andromonoecious individuals), except for one monoecious individual, contained the MSR and were OGI+; these findings strongly suggested that OGI is required for male tissue production in general sex determination of D. oleifera, as in monoecious D. kaki [17]. Among the co-sexual plants, the ability to produce male flowers was variable (Table S13), and thus the four exceptional gynoecious plants possessing OGI were considered female-biased monoecious plants. We did not include plants with inconsistent phenotypes (four gynoecious with OGI) in subsequent analyses.
Several individuals showed distinct flower sexes and morphologies, which comprised pseudo-monoecy. The pseudo-monoecious trees, lacking the MSR and OGI, form two- or three-flower cymes with a female (or occasionally hermaphroditic) flower in the middle and one or two abnormal small female flowers at the sides (Fig. 1D; Fig. S3).
In D. kaki, the OGI mRNA expression levels in both female and male floral buds of D. kaki were very low during development, which was attributed to the presence of Kali (a short interspersed nuclear element [SINE]-like insertion) on the OGI promoter [10]. However, in this study, none of the OGI+ D. oleifera individuals harboured Kali on the promoter of OGI (Method S5; Fig. S19). OGI is a pseudo-gene, which encodes small RNAs (smRNAs) [8]. Thus, the accumulation patterns of smRNAs on OGI gene were used to represent the OGI expression levels here. The abundance of smRNAs on OGI gene was significantly higher in male floral buds obtained from all the androecious, monoecious and andromonoecious D. oleifera trees (OGI+ individuals) than that in the female floral buds obtained from gynoecious trees (OGI− individuals) (Fig. 2A, C and D), which suggest that OGI is not seriously suppressed in D. oleifera. Therefore, previous molecular genetic knowledges for monoecious production in D. kaki might be not directly applicable to D. oleifera.
MeGI expression and smRNA accumulation in D. oleifera. A-E smRNA accumulation on OGI/MeGI genomic sequences in (A) female and male floral buds in dioecy; B stems of female and male shoots in dioecy; C female and male floral buds in monoecy; D male and hermaphroditic floral buds in andromonoecy; and E solitary floral buds and lateral floral buds of flower cymes in pseudo-monoecy. F Fragments per kilobase of transcript per million mapped reads (FPKM) values of MeGI in floral buds and stems. Data are means ± standard errors (three biological replicates) except for floral buds in pseudo-monoecious trees, for which no biological replicates were available
Different profiles of smMeGI abundance and MeGI transcripts between single-sexual and co-sexual D. oleifera
We investigated the accumulation patterns of smRNAs on OGI and MeGI regions (smMeGI) in D. oleifera (Fig. S19A); such patterns coincide with flower sex in D. lotus and D. kaki [8, 10]. In the single-sexual (gynoecious and androecious plants) D. oleifera, greater accumulation of smMeGI was detected in floral buds from androecious plants (androecious male; A_M) than in floral buds from gynoecious plants (gynoecious female; G_F) (Fig. 2A), similar to findings in D. lotus and D. kaki [10]. A similar pattern was observed in the immature stem tissues of flowering shoots (Fig. 2B). smMeGI degrade MeGI transcripts, thus as an expected consequence of the level of smMeGI, the level of MeGI transcripts was significantly higher in G_F than in A_M in mid-April (Fig. 2F), which was in accordance with the patterns in D. lotus and D. kaki [8, 10].
In contrast, the smMeGI patterns in the co-sexual types were distinct from the findings in previous reports. In the monoecious D. oleifera, where individuals exhibited separate male and female flowers, smMeGI accumulation was not significantly different between the female (monoecious female; M_F) and male floral buds (monoecious male; M_M) (Fig. 2C). Similarly, the level of MeGI expression was not significantly different between M_F and M_M; it was comparable with the level in male flower buds of androecious plants (A_M) (Fig. 2F).
In the hermaphroditic flowers, we observed an intermediate level of smMeGI. Among andromonoecious plants, the level of smMeGI accumulation was significantly lower in hermaphroditic floral buds (andromonoecious hermaphroditic; AM_H) than in male floral buds (andromonoecious male; AM_M) (Fig. 2D). In contrast to female flowers (G_F), where smMeGI was nearly absent, a substantial amount of smMeGI accumulation was detected in hermaphroditic flowers (AM_H) (Fig. 2A and D). The levels of MeGI expression were not significantly different between AM_M and AM_H (Fig. 2F).
In the OGI― pseudo-monoecious plants, low smMeGI accumulation was observed in both solitary female floral buds (pseudo-monoecious solitary female; PM_SF) and lateral floral buds of the three flower cymes (pseudo-monoecious lateral female; PM_LF) (Fig. 2E). MeGI expression was higher in PM_SF and PM_LF than in male floral buds from androecious, monoecious, and andromonoecious plants, consistent with the level of gynoecia development (Fig. 2F).
DNA methylation in D. oleifera floral buds and stems
In monoecious D. kaki, a lower methylation level of the MeGI promoter is associated with female flower formation in genetically male plants [10]. We evaluated whether this mechanism is active in the co-sexual D. oleifera, where sex expression does not match with the component of OGI/MeGI system. The DNA methylation levels of floral buds and stems were analysed by whole-genome bisulphite sequencing (Text S3; Tables S14-S19; Figs. S20-S21).
Unexpectedly, the DNA methylation patterns of the MeGI region in diverse D. oleifera matched the DNA methylation patterns of D. kaki and D. lotus, even in the co-sexual types. The methylation levels of the 5’-untranslated region and exon of MeGI were significantly lower in female floral buds than in male floral buds obtained from monoecious plants (Fig. 3A). This pattern was also observed in andromonoecious and single-sexual (gynoecious and androecious individuals) plants, which showed lower methylation levels in female tissues (AM_H, G_F, and G_S) than in male tissues (AM_M, A_M, and A_S) (Fig. 3A; Figs. S22 and S23).
DNA methylation in flower buds from co-sexual D. oleifera. A Methylation levels of the MeGI genomic region in floral buds from monoecious and andromonoecious D. oleifera. B-D Whole-genome comparison of methylation levels in the B CG, C CHG, and D CHH subcontexts between M_F and M_M. Tracks from outside to inside: methylation level of M_F; different methylation levels between M_F and M_M, where red and blue represent higher and lower methylation levels in M_F than in M_M, respectively; methylation level of M_M. Dolunmap and Chlor in (A), (B) and (C) represent the male-unmapped sequences and chloroplast genome, respectively
We next speculated that the discrepancy between the smMeGI level and DNA methylation in the co-sexual plants is related to changes in the global methylation pattern. Notably, the DNA methylation levels of floral buds in the CG, CHG, and CHH subcontexts were lower in female tissues (M_F) than in male tissues (M_M) in almost all genomic regions (Fig. 3B, C, and D). The DNA methylation levels in the CG and CHG subcontexts in every part of gene body, as well as the surrounding regions, were generally lower in M_F than in M_M (Fig. S24A and B). Most of the differentially methylated regions were distant from genes, although there were numerous differentially methylated regions in the promoter, exon, and intronic regions (Fig. S24C, D, and E). A similar pattern was observed in the single-sexual plants; the genome-wide DNA methylation levels in the CG, CHG, and CHH subcontexts were generally lower in females (G_F) than in males (A_M) (Fig. S25). The same trend was observed in stems of flowering shoots (Fig. S26). This finding was also observed for hermaphrodite flower formation. In andromonoecious plants, the DNA methylation levels of floral buds in all three subcontexts were lower in hermaphrodites (AM_H) than in males (AM_M) (Fig. S27). Therefore, female tissues showed lower global DNA methylation levels than male tissues in all sexual types, implying that a genome-wide decrease in DNA methylation promotes the development of gynoecia in both co- and single-sexual systems.
As a potential regulator of DNA methylation, we investigated the expression levels of DNA methyltransferase/demethylase genes by RNA-Seq. A demethylase gene (evm.model.Chr7.1501), homologous to REPRESSOR OF SILENCING1 (ROS1) [18], was downregulated in AM_M compared with AM_H (Table S20), possibly explaining the dynamic modulation of genome-wide methylation levels in andromonoecious D. oleifera.
Overlap of mRNA-miRNA functional modules between co- and single-sexual systems
To characterise the functional overlap of gynoecia/androecia development in different sexual expression systems, transcriptome analyses were conducted using 26 samples of floral buds and stems of flowering shoots (Text S4; Figs. S28-S37; Tables S21-S26). We found common sexually distinct regulatory networks of microRNAs (miRNAs) and their targets, as well as common functional enrichments for gynoecia development in single- and co-sexual systems.
Based on the interaction network analysis and functional annotation, highly expressed miRNAs in female (or hermaphroditic) and their mRNA targets downregulated in female (or hermaphroditic) floral buds, and vice versa (i.e., low-level miRNA and high-level mRNA targets in females), were identified in single- and co-sexual systems (Fig. 4; Tables S25 and S26). At least two of the three male-active networks (low-level miRNA and high-level mRNA targets in male tissues) (Fig. 4A, B, and C) included the exonuclease mut-7 homolog, NOZZLE, GAMYB (GAM1), auxin response factor 18 family members, cinnamoyl-CoA reductase 2, myosin-11, and evm.model.Chr12.1832.1. Specifically, NOZZLE and GAM1 were detected in all three networks. In contrast, at least two of the three female-active networks (low-level miRNA and high-level mRNA targets in female tissues) (Fig. 4D, E, and F) included squamosa promoter-binding-like protein gene 7 (SPL7), SPL9, SPL16, SPL17, lysine-specific demethylase (JMJ25), BHLH25, PCS1, MAR-binding filament-like protein 1–1 (MFP1-1), origin of replication complex subunit 1A (ORCS-1A), Fanconi anaemia group M protein homolog, and cucumisin, growth-regulating factor 6 (GRF6), ORCS-1A, ATP sulphurylase 1-chloroplastic, and SOBIR1.
Networks constructed based on differentially expressed miRNAs and their mRNA targets, as well as KEGG pathway enrichments of differentially expressed genes. miRNAs with higher expression and their downregulated targets (A) in G_F compared with A_M, (B) in M_F compared with M_M, (C) in AM_H compared with AM_M, (D) in A_M compared with G_F, (E) in M_M compared with M_F, and (F) in AM_M compared with AM_H. KEGG pathway enrichments [19,20,21] of (G) up- and (H) downregulated genes in female (or hermaphroditic) floral buds compared with male floral buds
Comparisons of female (or hermaphroditic) and male tissues revealed that numerous Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were commonly enriched in both single- and co-sexual systems, including the female-active pathways phenylpropanoid biosynthesis, biosynthesis of secondary metabolites, and plant hormone signal transduction, etc.(Fig. 4G), as well as the male-active pathways starch and sucrose metabolism and galactose metabolism, etc.(Fig. 4H) [19,20,21]. Therefore, it was suggested that single- and co-sexual systems use the same basic functional modules for flower sex determination.
Core gene networks correlated with sex differentiation in female and male floral buds
To evaluate the regulatory paths of sex differentiation in female and male floral buds in co- and single-sexual systems, coexpression patterns were visualised by weighted correlation network analysis using all female and male floral buds that had been subjected to RNA-Seq analysis (Table S27). A scale-free topology model fit soft threshold of 7 (Fig. S38A and B) was applied and the coexpression pattern was clustered into 21 modules (Fig. S38C and D).
The midnight-blue and yellow modules showed the strongest positive correlations with male expression, and genes in these modules may promote the development of male floral buds (Fig. S38E). The midnight-blue module contained 156 genes, which were significantly (corrected P < 0.05) enriched in five GO terms: regulation of transcription DNA-templated, regulation of primary metabolic process, regulation of gene expression, regulation of cellular macromolecule biosynthetic process, and copper ion binding (Fig. S39A). The yellow module contained 378 genes, which were significantly enriched in the organic substance transport GO term (Fig. S39B).
The pink and green modules showed the strongest positive correlations with female expression, and genes in these modules may promote the development of female floral buds. The pink module contained 285 genes, which were significantly enriched in five GO terms: UDP-glycosyltransferase activity, transferase activity, transferring glycosyl groups, single-organism process, single-organism metabolic process, and oxidoreductase activity (Fig. S39C). Genes in the pink module were also significantly enriched in two KEGG pathways: phenylpropanoid biosynthesis and linoleic acid metabolism (Fig. S39D).
Genes with top 20 connections were identified in the midnight-blue, yellow, pink, and green modules, and used to construct networks, respectively (Fig. 5). Transcription factors (TF) in the networks were highlighted. Heat stress TF B-3 (HSFB3) in the midnight-blue module and B3 domain-containing transcription repressor (VAL1) in the yellow module were shown to be potential key genes for male development (Fig. 5A and B). HSFB3 showed sharply higher expression levels in male floral buds than in female in both dioecious and monoecious plants (Fig. 5E), supporting a potential male promoting effect. Although GAM1 was identified in the pink module which was positively correlated with female development, the expression levels of GAM1 were slightly higher in male floral buds than that in female (Fig. 5F), suggesting this gene may promote male development. This is in accordance with the results shown in the mRNA-miRNA functional modules mentioned above. MADS-box protein JOINTLESS (J), Myb-related protein B (myb12) and bHLH96 (BHLH96) in the green module were shown to be potential key genes for female development (Fig. 5 C and D), which was further demonstrated by the results that all these genes were higher expressed in the female floral buds than in male in both dioecious and monoecious plants (Fig. 5G, H and I).
Networks constructed with genes harbored top 20 connections in the (A) midnight-blue, (B) yellow, (C) pink, and (D) green modules, respectively. FPKM values of (E) HSFB3, (F) GMA1, (G) myb12, (H) BHLH96 and (I) MADS-box JOINTLESS in floral buds and stems. Gene names in red in (A), (C) and (D) represent TFs. The rectangle color from dark red to light yellow in (A-D) represents a decreasing trend of connectivity. Data are means ± standard errors (three biological replicates) except for floral buds in pseudo-monoecious trees, for which no biological replicates were available in (E-I)
Population structure in D. oleifera according to sexual expression
We performed whole-genome sequencing of 150 D. oleifera individuals with a mean depth of 20 × . After filtering, 3,545,359 single nucleotide polymorphisms (SNPs) and 318,863 indels were obtained for further analysis. The mean PI_HAT value was 0.056, indicating a low level of familial relationship in the population. PCA showed that the sampled individuals could be divided into three clusters (Fig. 6A). Single-sexual plants and monoecious plants were found in all three clusters, whereas andromonoecious and androgynomonoecious trees were found only in groups 2 and 3. Pseudo-monoecious plants were found only in group 2, and they are distributed in close proximity. The results imply that the monoecious genetic factor prevails in D. oleifera, whereas andromonoecious, androgynomonoecious, and pseudo-monoecious individuals may be rare and develop only under certain conditions. The maximum-likelihood phylogenetic tree supported this notion; individuals obtained in close areas tended to have a close genetic relationship (Fig. 6B). The decay of linkage disequilibrium (LD) with physical distance between SNPs occurred at < 200 bp (r2 = 0.2) (Fig. S40A).
Phylogeny of the 150 D. oleifera plants. A Scatter clustering diagram based on the first two principal components after PCA of whole-genome sequence data. PC1 and PC2 explained 7.42% and 5.59% of the total variance, respectively. B Maximum-likelihood phylogenetic tree of the 150 D. oleifera plants using MEGA-X labelled in order of sampling time. Therefore, similar numbers indicate relatively close distributions
GWAS of the co-sexual phenotypes
To identify the genomic factors that confer the monoecious phenotype, we performed GWAS for the proportion of female shoots in genetically male plants. Ninety individuals with male flower production were selected; and 3,502,197 SNPs and 311,512 indels were retained for further analysis after LD pruning (Table S28).
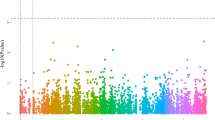
GWAS for the proportion of female shoots (Table S13) detected a significant peak on chromosome 7 (Fig. 7A and S40B). Individuals with a heterozygous genotype at the locus with the strongest association signal showed greater proportions of female shoots (Fig. 7B; Table S29). A haplotype block spanning 29.0–29.4 Mb on chromosome 7 was strongly associated with phenotype (Fig. 7A). Seven genes of the DUF247 family were identified in this block (Fig. 7C). Most were upregulated in female tissue compared with male tissue; one of these genes, evm.model.Chr7.983, was significantly upregulated in female floral buds compared with male floral buds in both monoecious and single-sexual plants (Fig. 7D; Table S30). Most of the variants with the highest peak association were distributed upstream of evm.model.Chr7.983 (Fig. 7C), which may contribute to the differential expression of this gene. miRNA pab-miR3711, located within the block spanning 29.0–29.4 Mb, was downregulated in female floral buds compared with male tissues in monoecious plants (Table S31), possibly in relation to monoecious phenotype development.
GWAS for the monoecious phenotype in D. oleifera. A Manhattan plot for the proportion of female shoots in 90 plants with male flower production (top), along with a local Manhattan plot and LD heatmap (bottom) of the associated region on chromosome 7. B Proportion of female shoots based on genotype at the most significant locus, Chr7: 29256366. C Schematic representation of gene position in the 29.0–29.4 Mb region of Chr7. D Expression pattern of genes in the 29.0–29.4 Mb region of Chr7 in female (or hermaphroditic) and male floral buds in single- and co-sexual systems. Red line in (A) represents the Bonferroni-corrected P-value of 0.05, as shown in Figs. 7A and 8A. Chromosome 16 in (A) represents the male-unmapped sequences, which is consistent in the following figures
We also recorded the proportion of hermaphroditic floral branches in the 90 plants. GWAS analysis for the proportion of hermaphrodite shoots revealed strong signals on chromosomes 2, 11, and 14 (Fig. 8A). According to the LD analysis, five, two, and three LD blocks with strong associations were detected on chromosomes 2, 11, and 14, respectively (Fig. S41). Individuals with heterozygous genotypes at the loci with the strongest association signals showed greater proportions of hermaphroditic shoots for all detected blocks (Fig. 8B and C; Table S32). In total, 65 genes were identified on the blocks, among which 3 and 2 genes were up- and downregulated in male floral buds compared with hermaphroditic floral buds in andromonoecious plants, respectively (Fig. 8D; Table S33). Additionally, genes encoding 31 lncRNAs (Table S34), and 9 miRNAs (Table S35) were identified in these regions. Further analysis of these sequences may identify genetic events linked to hermaphroditic flower development in genetically male D. spp.
GWAS for hermaphroditic flower development in D. oleifera. A Manhattan plot for the proportion of hermaphroditic shoots among 90 samples with male flower production. B Proportion of hermaphroditic shoots based on genotype at the most significant locus, Chr14: 31311437, and C the second most significant locus, Chr2: 30821709. D Genes with significantly different expression in AM_M compared with AM_H in the association regions
Absence of the YY genotype in the D. oleifera population
The emergence of co-sexual phenotype enables crossing among genetically male plants. A model-based analysis showed that the stability of dissolution of dioecy depends on the viability of the YY genotype [22], which is reduced by loss of function of Y-linked genes. Therefore, we characterised the sex-linked region. GWAS for the male expression (or OGI+) (Table S13) of 150 plants identified a sex-linked region at 22.0–32.0 Mb on chromosome 4 (Figs. 9A and S42-43; Table S36). This region corresponds to the male-specific region in D. lotus [16] (Fig. 9B). The genotype of the SNPs with the strongest association signals in this region showed that most of the 54 gynoecious individuals (OGI−) were homozygous (XX type), whereas most of the 89 male-functional individuals were heterozygous (XY type) (Fig. 9C; Table S37). Genoty** analysis indicated that the YY genotype was not supported by > 2 successive variant loci (Table S41). The occasional YY genotype may be attributed to recombination or genoty** error associated with the highly repetitive nature of this region. Therefore, the YY genotype is either present at a negligible level or absent from the population.
Genetic characterisation of the sex-linked region in the D. oleifera population. A GWAS for male expression. B Alignment of Chr4 of D. oleifera and Chr15 of D. lotus. C Genotype fractions of the SNPs with strong association signals in the sex-linked region. D Differentially expressed genes in the sex-linked region
We detected a potential X-specific region and putative sex-related genes in this region, in addition to the male determinant OGI (Text S5; Table S38). Twenty-seven genes were differentially expressed between female (or hermaphroditic) and male floral buds in single- or co-sexual systems, or both (Fig. 9D). Specifically, two genes, GLO and ARR9, which have masculinising functions in Antirrhinum majus [23] and feminising functions in the genus Populus [24], respectively, were differentially expressed in this region (Fig. 9D).
Discussion
A sex-linked region contributing to sex dimorphism and diversity
All D. oleifera trees with male function, but exceptional one monoecious individual, had the MSR and OGI (Table 1), and they showed many heterozygous genotypes in the sex-linked region on chromosome 4 (Fig. 8), consistent with the male heterogametic (XY) system. smMeGI and MeGI transcript analyses implied that the OGI/MeGI system [8] functions in the sex determination of single-sexual D. oleifera plants (Fig. 2).
In the D. oleifera population, two types of co-sexual systems were identified: monoecy and hermaphroditic flower formation. The genoty** results (Table 1) indicate that all individuals can be divided into two groups: a genetically female (XX) group that includes gynoecious and pseudo-monoecious plants, and a genetically male (XY) group that includes androecious, monoecious, androgynomonoecious, and andromonoecious plants. Thus, the co-sexual types (monoecious, androgynomonoecious, and andromonoecious) may represent results of the breakdown of dioecy, where androecious trees acquire gynoecia development functions as demonstrated empirically in various species, including grape and papaya [5, 22, 25,26,27].
The lack of YY individuals in this study (Fig. 8) was surprising to us because it was inconsistent with the potential for crossing among genetically male individuals, considering that flowering times of male and female flowers in D. oleifera (and D. kaki) usually fully overlapped. This finding may be attributed to the low viability of the YY genotype (i.e., genetic degeneration). Genetic modelling has shown that under such conditions, stable coexistence of single- and co-sexual plants in a population (subdioecy) can be achieved, because it is likely that Y chromosome has not yet evolved to fully suppress female functions [22]. In the present study, genomic and transcriptional analyses identified putative Y-/X-specific regions (Fig. 8C), as well as several genes in the sex-linked region that are differentially expressed between female and male tissues and may function in sexual expression, such as GLO and ARR9 (Text S5); these findings imply functional divergence of the X and Y chromosomes. GLO is reportedly essential for stamen development in Antirrhinum majus [23], whereas ARR proteins function in gynoecia development and are regarded as master regulators of sex expression in the genus Populus [24]. The sex-linked region and the essential genes within that region presumably cooperate with the OGI/MeGI system to regulate the sex differentiation and diversity of D. spp.
Feminising scenario that contribute to the dissolution of dioecy in co-sexual D. oleifera
We identified three potential feminising mechanisms in both single- and co-sexual systems: increased expression of the feminising gene MeGI and decreased abundance of smMeGI; genome-wide decrease in methylation levels; and sexual distinct regulatory networks of smRNAs and their targets. However, the first mechanism was inconsistent in the monoecious system; MeGI expression and smMeGI accumulation were not significantly different between M_F and M_M (Fig. 2). This is inconsistent with the pattern in monoecious hexaploid D. kaki, which has lower smMeGI levels and higher MeGI levels in the female flower buds of genetically male plants [10]. Considering the similar MeGI expression in monoecious (both male and female floral buds) and androecious (male floral buds) plants (Fig. 2F), monoecious D. oleifera may develop gynoecia independently of MeGI regulation.
The mechanism that underlies gynoecia development in monoecious plants may be global regulation of DNA methylation (Fig. 3). Treatment of male flower buds with the DNA methylation inhibitor zebularine and 5-azacytidine induces pistil development and reduces pollen fertility in some D. kaki cultivars [10, 28]. The DNA demethylase gene ROS1 [18] was upregulated in hermaphroditic floral buds compared with male floral buds in andromonoecious D. oleifera (Table S20), which may explain the genome-wide decrease in methylation level in hermaphroditic floral buds compared with male floral buds.
We also identified several putative key relationships between miRNA expression and target expression. Decreased expression of GAMYBs (evm.model.Chr13.1162 and evm.model.Chr11.1032) and increased expression of putative miRNA regulators were identified in female (or hermaphroditic) floral buds compared with male floral buds. GAMYB is a trans-activator of GA signalling [29, 30] and functions in flower development [31, 32]. The upregulation of GAMYB in male floral buds implies that GA signalling promotes the development of androecious tissues, consistent with our previous findings that GA promotes the male function in monoecious [33, 34] and andromonoecious [12] D. kaki. Furthermore, SPL family genes (evm.model.Chr14.920, evm.model.Chr7.129, evm.model.Chr7.171, and evm.model.Chr12.765), JMJ25, and GRFs were commonly activated in female tissues, presumably through miRNA regulation. SPL family genes have diverse functions in plant development [35]; one of these genes acts a direct upstream activator of LEAFY, FRUITFULL, and APETALA1 to control the timing of flower formation [36]. JMJ25 is a histone H3K9 demethylase gene that reportedly affects DNA methylation [37]. GRFs are plant-specific transcription factors, and the miR396/GRF regulatory network is required for the proper development of the pistil in Arabidopsis [38, 39]. The functions of these genes in model plants and their expression patterns in D. oleifera were consistent with the working hypothesis regarding sexual expression in D. oleifera.
Expression of heat stress TF B-3 (HSFB3) was reported to be firmly correlated with abiotic and biotic stress [40]. HSFB3 was sharply higher expressed in male floral buds than that in female, suggesting that when plants were suffering from abiotic or biotic stress, they tended to bear male flowers. Thus, protection against abiotic or biotic stress contributes to the feminising scenario in D. oleifera.
It is worth noting that samples used for methylome and transcriptome analyses were obtained in mid-Apil, when the pistil or stamen primordia inside persimmon floral buds were alternatively arrested, leading to the final sex expression [9]. Thus, the feminising scenario uncovered at this developmental stage should be a part of sex determination system regulated by the upstream genetic factors.
Genetic factors linked to the dissolution of dioecy
The monoecious phenotype in D. oleifera was unique and could not be well explained by known mechanisms. Therefore, we evaluated the genetic mechanisms that underlie the sexuality of monoecious and andromonoecious types. The candidate region for the monoecious trait on chromosome 7 included a cluster of seven DUF247 genes (Fig. 6). The Y-specific dominant female suppression gene, SOFF, in dioecious Asparagus officinalis is a member of this gene family [41]. In A. officinalis, knockout of the SOFF gene converts males to hermaphrodites, knockout of the Y-specific male-promoting aspTDF1 converts males to neuters, and knockout of both TDF1 and SOFF converts males to females [42]. A DUF247 family gene was identified as a male component of the self-incompatibility S-locus in perennial ryegrass [43, 44]. We detected clusters of duplicated DUF family genes in at least five loci in the D. oleifera genome (data not shown), implying functional divergence. The monoecious determinant, as well as its molecular genetic control, must be identified in subsequent studies. Although a very strong signal was obtained for chromosome 7, it could not explain all monoecious phenotypes (Fig. 6B), implying that other loci and environmental factors affect female development.
The development of hermaphroditic flowers in dioecious systems because of mutations at the sex-determining locus has been observed in grape and papaya [26, 27, 45]. In contrast, our GWAS approach for the differentiation of hermaphroditic floral buds yielded candidate regions on chromosomes 2, 11, and 14, but not on chromosome 4 (which has the sex-linked region). We also observed decreased expression of the feminising gene MeGI in hermaphroditic flower buds (Fig. 2F). Therefore, the establishment of hermaphroditic flower development in D. oleifera is independent of direct activation/inactivation of the genetic regulation of sex dimorphism through the existing OGI/MeGI system, as implied in work regarding D. kaki [7, 12].
Masuda et al. (2022b) reported that DkRAD regulates gynoecia formation in hermaphroditic flowers of hexaploid D. kaki [7]. This may also function in D. oleifera because the expression of the DkRAD homologue was higher in female and hermaphroditic tissues than in male tissues (Fig. S44), as in D. kaki. One discrepancy compared with the work of Masuda et al. (2022b) [7] is that our study revealed many diploid D. oleifera plants bearing hermaphroditic flowers, whereas Masuda et al. regarded the hermaphrodite mechanism in Diospyros as polyploid species-specific. Our results indicate that the evolution of hermaphroditic flower development in Diospyros is not ploidy-dependent; however, considering the similarity in sexual expression between D. kaki and D. oleifera, as well as their close phylogenetic relationship [46], a common evolutionary event and mechanism presumably led to sex expression diversity in both species. Further genetic analyses of sex expression in D. kaki and D. oleifera are needed.
Summary and future perspectives
Based on our findings for D. oleifera, female shoots mainly develop from mixed dormant buds that developed on the tips of the flowering mother branches in monoecious plants which are genetically male with OGI. In contrast, male shoots mainly develop on the basal parts of the mother branches (Fig. 10). Floral primordia in D. oleifera are initiated in early summer, then experience a long dormant period until the following spring to break the buds [9]. Therefore, dormant floral buds on the tips of the flowering mother branches are more likely to activate the feminising scenario under natural conditions (Fig. 10). The same pattern was observed in cultivated hexaploid D. kaki (Fig. S45). An investigation considering arrangement of sex within flowering mother branches will further expand the understanding of physiological and molecular basis of sex expression in Diospyros.
Conclusions
Co-sexual expression (monoecy and hermaphroditic development), previously thought to be polyploid-specific in Diospyros species, was identified in the diploid D. oleifera historically. We characterized potential genetic mechanisms that underlie the dissolution of dioecy to monoecy and andro(gyno)monoecy, based on multiscale genome-wide investigations of 150 accessions of Diospyros oleifera. We found all co-sexual plants, including monoecious and andro(gyno)monoecious individuals, possessed the male determinant gene OGI, implying the presence of genetic factors controlling gynoecia development in genetically male D. oleifera. In both single- and co-sexual plants, female function was expressed in the presence of a genome-wide decrease in methylation levels, along with sexually distinct regulatory networks of smRNAs and their targets. Furthermore, a genomic region and a DUF247 gene cluster strongly associated with the monoecious phenotype and several regions that may contribute to andromonoecy were identified. Collectively, our findings demonstrate stable breakdown of the dioecious system in D. oleifera, presumably also a result of genomic features of the Y-linked region.
Methods
Plant material and phenoty**
A D. oleifera collection from Guilin, Guangxi Zhuang Autonomous Region, China, was evaluated for sexual expression in the 2019 and 2021 seasons. Two hundred eight D. oleifera trees were found in the natural population. In D. oleifera, the flower sex on a single shoot is uniform. Thus, for simplicity and accuracy, large flowering mother branches (approximately 1.5 m in height × 1.5 m in width) containing ≥ 20 flowering shoots (each uniformly bearing female, male, or hermaphroditic floral buds) were used to calculate the proportions of female, male, and hermaphroditic shoots in monoecious, androgynomonoecious, and andromonoecious trees. At least five large flowering mother branches of each tree were selected; the highest female and hermaphroditic shoot proportions of the large flowering mother branches were used for GWAS as an indication of feminising ability on the co-sexual tree.
Reference sequence construction
The female plant ‘D. oleifera 1’ was used to optimise the published version of the D. oleifera genome [47] using a BioNano optical map**-assisted assembly (Method S2), resulting in a new D. oleifera main genome. This genome was deposited in figshare (https://doi.org/10.6084/m9.figshare.20101664.v3) [15]. The male-specific region (the MSR) was absent in this genome. Thus, the resequencing reads of 14 androecious, 4 andromonoecious, 15 monoecious, 7 androgynomonoecious, and 2 pseudo-monoecious D. oleifera individuals (detail information of resequencing reads was introduced in the following text) were mapped to the D. oleifera main genome with the Burrows-Wheeler Aligner (BWA) mem option and the paired-end model [48]. The unmapped reads were extracted using SAMtools [49], and they were assembled using SoapDenovo [50] to construct the male-unmapped sequences (Methods S3-S4), which was deposited in figshare (https://doi.org/10.6084/m9.figshare.20407386.v1) [51]. The D. oleifera main genome [15], male-unmapped sequences [51], and the D. oleifera chloroplast genome [46] were combined as a reference genome for methylome, whole-transcriptome, and resequencing analyses.
Methylome and transcriptome analyses
Floral bud and immature stem tissues of flowering shoots were sampled in mid-April (April 15–17th), 2019, which is a key period for the differentiation of flower sex types (Method S6). Those samples (Table 2) were used for the whole-genome bisulphite sequencing, transcriptome sequencing, and small RNA (smRNA) sequencing. All reads obtained were mapped to the combined reference D. oleifera genome. Details of library construction, sequencing, and analysis are provided in Methods S7-S9.
The smRNAs-Seq reads were also mapped to the OGI sequence from the D. lotus genome [16], MeGI sequence from the D. oleifera reference genome, and the ‘Kali’ sequence reported by [10], using the method established by Akagi et al. (2016a) [10]. Here, the smRNAs-Seq reads mapped onto the MeGI gene body were referred to as smMeGI. The accumulation levels of smMeGI and each fragment were recorded as reads per million reads. Two independent-samples T test (SPSS, Inc, Chicago, IL, USA) was used to determine the significant difference between female (or hermaphroditic) and male floral buds (or stems) in each sexual systems.
Resequencing analysis
A set of 150 D. oleifera (Table 1; Table S13) was selected from the collection and used for resequencing analysis. Short read resequencing (PE150) by the Illumina NovaSeq 6000 platform yielded 3.03 Tb of raw data with ~ 20-fold genomic coverage for each sample. All reads were firstly mapped to the D. lotus OGI genomic sequence and the D. lotus the MSR sequence [16] using the Bwa mem option and the paired-end model to check whether each D. oleifera individual was OGI-positive or not. Subsequently, all the reads were mapped the combined D. oleifera reference genome using the Bwa mem option and the paired-end model. SAMtools and the Genome Analysis Toolkit (version 2.4–7-g5e89f01) were used to label SNPs and insertion-deletions (indels). Polymorphisms that matched the following four criteria were filtered out: > 2 alleles, variants beyond the read depth between half and twice the genome-wide average, missing rates ≥ 0.25, and minor allele frequency < 0.05. The linkage disequilibrium (LD) was evaluated using the pairwise squared Pearson’s correlation coefficient (r2) calculated by PLINK version 1.9 [52]. LD pruning was conducted by a standard method (PLINK –indep 50 5 2).
Using the filtered variant sets, the following analyses were conducted. First, the familial relationships and sample uniqueness were evaluated based on the PI_HAT value computed by PLINK. Then the population structure of the 150 trees was estimated using principal component analysis (PCA) in EIGENSTRAT software [53]. The maximum-likelihood phylogenetic tree was constructed using MEGA-X [54] with 1000 replicates using the following parameters: gaps/missing data, partial deletion; site coverage cut-off, 90%; general time reversible model; and rates among sites, uniform.
GWAS
GWAS was performed using the linear mixed model in the R package rrBLUP [55]. A kinship (K) matrix (generated with the A.mat function of rrBLUP) was included in the linear mixed model, along with 6 principal components (PCs) for 90 individuals with male production (Fig. S40B) and 4 PCs for all 150 individuals (Fig. S42). Bonferroni correction (corrected P < 0.05) was used to determine the genome-wide significance thresholds. The LD patterns surrounding GWAS peaks were visualised using the R package LDheatmap [56] for chromosome 7, and using Haploview (http://www.broadinstitute.org/haploview) for chromosomes 4, 2, 11, and 14. The regions with pairwise r2 > 0.5 were regarded as candidate LD blocks.
Availability of data and materials
The raw sequence data of DNA resequencing, BS-seq, smRNA and BioNano molecules that support the findings of this study are openly available in the Genome Sequence Archive (GSA, https://ngdc.cncb.ac.cn/gsa/) with accession numbers of CRA007608, CRA007609, CRA007611, CRA007613, respectively. The raw sequence data of lncRNA that support the findings of this study are openly available in the Genome Sequence Archive (GSA, https://ngdc.cncb.ac.cn/gsa/) with an accession number of CRA007610 as reported by Mai et al. (2022) [57].
Abbreviations
- A_M:
-
Androecious male floral buds
- A_S:
-
Andromonoecious stems of immature flowering shoots
- AM_H:
-
Andromonoecious hermaphroditic floral buds
- AM_M:
-
Andromonoecious male floral buds
- BWA:
-
Burrows-Wheeler Aligner
- FPKM:
-
Fragments per kilobase of transcript per million mapped reads
- GAM1 :
-
GAMYB
- GRF6 :
-
Growth-regulating factor 6
- GWAS:
-
Genome-wide association study
- G_F:
-
Gynoecious female floral buds
- G_S:
-
Gynoecious stems of immature flowering shoots
- HSFB3 :
-
Heat stress TF B-3
- indels:
-
Insertion-deletions
- J :
-
MADS-box protein JOINTLESS
- JMJ25 :
-
Lysine-specific demethylase
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LD:
-
Linkage disequilibrium
- MFP1-1 :
-
MAR-binding filament-like protein 1–1
- miRNAs:
-
MicroRNAs
- the MSR:
-
Male-specific region
- myb12 :
-
Myb-related protein B
- M_F:
-
Monoecious female floral buds
- M_M:
-
Monoecious male floral buds
- OGI − :
-
OGI-Negative
- OGI + :
-
OGI-Positive
- ORCS-1A :
-
Origin of replication complex subunit 1A
- PCA:
-
Principal component analysis
- PM_LF:
-
Pseudo-monoecious lateral female floral buds
- PM_SF:
-
Pseudo-monoecious solitary female floral buds
- ROS1 :
-
REPRESSOR OF SILENCING1
- smMeGI :
-
Accumulation patterns of smRNAs on OGI and MeGI regions
- smRNA:
-
Small RNA
- SNPs:
-
Single nucleotide polymorphisms
- SPL7 :
-
Squamosa promoter-binding-like protein gene 7
References
Renner SS, Ricklefs RE. Dioecy and its correlates in the flowering plants. Am J Bot. 1995;82:596–606.
Weiblen GD, Oyama RK, Donoghue MJ. Phylogenetic analysis of dioecy in monocotyledons. Am Nat. 2000;155:46–58.
Renner SS. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am J Bot. 2014;101:1588–96.
Heilbuth JC. Lower species richness in dioecious Clades. Am Nat. 2000;156(3):221–41.
Kafer J, Marais GAB, Pannell JR. On the rarity of dioecy in flowering plants. Mol Ecol. 2017;26:1225–41.
Badouin H, Velt A, Gindraud F, Flutre T, Marais GA. The wild grape genome sequence provides insights into the transition from dioecy to hermaphroditism during grape domestication. Genome Biol. 2020;21(1):223–223.
Masuda K, Ikeda Y, Matsuura T, Kawakatsu T, Tao R, Kubo Y, Ushijima K, Henry IM, Akagi T. Reinvention of hermaphroditism via activation of a RADIALIS-like gene in hexaploid persimmon. Nat plants. 2022;8:217–24.
Akagi T, Henry IM, Tao R, Comai L. A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science. 2014;346:646–50.
Li JR, Sun P, Han WJ, Li FD, Fu JM, Diao SF. Morphological key period study on floral sex differentiation in pollination-constant and non-astringent persimmon ‘Zenjimaru.’ Acta Horticulturae Sinica. 2016;43:451–61 (In Chinese with English abstract).
Akagi T, Henry IM, Kawai T, Comai L, Tao R. Epigenetic regulation of the sex determination gene MeGI in polyploid persimmon. Plant Cell. 2016;28:2905–15.
Wang L, Li H, Sun P, Suo Y, Han W, Diao S, Mai Y, Li F, Fu J. Efects of plant growth regulators, soil moisture contents, and carbon/nitrogen ratios on sex diferentiation in Persimmon (Diospyros kaki Thunb.) Flowers. J Plant Growth Regul. 2021;40:1121–38.
Li H, Wang L, Mai Y, Han W, Suo Y, Diao S, Sun P, Fu J. Phytohormone and integrated mRNA and miRNA transcriptome analyses and differentiation of male between hermaphroditic floral buds of andromonoecious Diospyros kaki Thunb. BMC Genomics. 2021;22:203.
Henry IM, Akagi T, Tao R, Comai L. One hundred ways to invent the sexes: theoretical and observed paths to dioecy in plants. Annu Rev Plant Biol. 2018;69(1):553–75.
Masuda K, Akagi T. Sexual system and its evolution. In: Tao R, Luo Z, editors. The Persimmon Genome. Cham: Springer Nature Switzerland AG; 2022. p. 97–108.
Sun P, Fu JM. A BioNano optical map**-assisted chromosomal genome assembly of Diospyros oleifera. figshare. Dataset. 2022a. https://doi.org/10.6084/m9.figshare.20101664.v3
Akagi T, Shirasawa K, Nagasaki H, Hirakawa H, Tao R, Comai L, Henry IM. The persimmon genome reveals clues to the evolution of a lineage-specific sex determination system in plants. PLoS Genet. 2020;16(2):e1008566.
Akagi T, Kawai T, Tao R. A male determinant gene in diploid dioecious Diospyros, OGI, is required for male flower production in monoecious individuals of Oriental persimmon (D. kaki). Sci Hortic-Amsterdam. 2016;213:243–51.
Gong Z, Morales-Ruiz T, Ariza RR, Roldan-Arjona T, David L, Zhu JK. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell. 2002;111:803–14.
Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–51.
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51:D587–92.
Crossman A, Charlesworth D. Breakdown of dioecy: models where males acquire cosexual functions. Evolution. 2014;68(2):426–40.
Perbal MC, Haughn G, Saedler H, Schwarz-Sommer Z. Non-cell-autonomous function of the Antirrhinum floral homeotic proteins DEFICIENS and GLOBOSA is exerted by their polar cell-to-cell trafficking. Development. 1996;122(11):3433–41.
Müller NA, Kersten B, Montalvão APL, Mähler N, Bernhardsson C, Bräutigam K, Lorenzo ZC, Hoenicka H, Kumar V, Mader M, Pakull B, Robinson KM, Sabatti M, Vettori C, Ingvarsson PK, Cronk Q, Street NR, Fladung M. A single gene underlies the dynamic evolution of poplar sex determination. Nat plants. 2020;6:630–7.
Cossard GG, Gerchen JF, Li X, Cuenot Y, Pannell JR. The rapid dissolution of dioecy by experimental evolution. Current Biol. 2021;31:1–7.
Massonnet M, Cochetel N, Minio A, et al. The genetic basis of sex determination in grapes. Nat Commun. 2020;11:2902.
VanBuren R, Zeng F, Chen C, Zhang J, Wai MC, Han J, Aryal R, Gschwend AR, Wang J, Na JK, Huang L, Zhang L, Miao W, Gou J, Arro J, Guyot R, Moore RC, Wang ML, Zee F, Charlesworth D, Moore PH, Yu Q, Ming R. Origin and domestication of papaya Yh chromosome. Genome Res. 2015;25(4):524–33.
Wang L, Li H, Suo Y, Han W, Diao S, Mai Y, Wang Y, Yuan J, Ye L, Pu T, Zhang Q, Sun P, Li F, Fu J. Effects of different chemicals on sexual regulation in persimmon (Diospyros kaki Thunb.) Flowers. Front Plant Sci. 2022;13:876086.
Gubler F, Kalla R, Roberts JK, Jacobsen JV. Gibberellin-regulated expression of a myb gene in barley aleurone cells: evidence for Myb transactivation of a high-pI alpha-amylase gene promoter. Plant Cell. 1995;7:1879–91.
Haseneyer G, Ravel C, Dardevet M, Balfourier F, Sourdille P, Charmet G, Brunel D, Sauer S, Geiger HH, Graner A, Stracke S. High level of conservation between genes coding for the GAMYB transcription factor in barley (Hordeum vulgare L.) and bread wheat (Triticum aestivum L.) collections. Theor Appl Genet. 2008;117:321–31.
Gocal GFW, Poole AT, Gubler F, Watts RJ, Blundell C, King RW. Long-day up-regulation of a GAMYB gene during Lolium temulentum inflorescence formation. Plant Physiol. 1999;119:1271–8.
Murray F, Kalla R, Jacobsen J, Gubler F. A role for HvGAMYB in anther development. Plant J. 2003;33:481–91.
Sun P, Li JR, Du GG, Han WJ, Fu JM, Diao SF, Suo YJ, Zhang Y, Li FD. Endogenous phytohormone profiles in male and female floral buds of the persimmons (Diospyros kaki Thunb.) during development. Sci Hortic-Amsterdam. 2017;218:213–21.
Li SZ, Sun P, Du GG, Wang LY, Li HW, Fu JM, Suo YJ, Han WJ, Diao SF, Mai YN, Li FD. Transcriptome sequencing and comparative analysis between male and female floral buds of the persimmon (Diospyros kaki Thunb.). Sci Hortic-Amsterdam. 2019;246:987–97.
Preston JC, Hileman LC. Functional evolution in the plant SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) gene family. Front Plant Sci. 2013;4:1–13.
Yamaguchi A, Wu MF, Yang L, Wu G, Poethig RS, Wagner D. The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev Cell. 2009;17:268–78.
Fan D, Wang X, Tang X, Ye X, Ren S, Wang D, Luo K. Histone H3K9 demethylase JMJ25 epigenetically modulates anthocyanin biosynthesis in poplar. Plant J. 2018;96:1121–36.
Omidbakhshfard MA, Proost S, Fujikura U, Mueller-Roeber B. Growth-regulating factors (GRFs): a small transcription factor family with important functions in plant biology. Mol Plant. 2015;8:998–1010.
Liang G, He H, Li Y, Wang F, Yu D. Molecular mechanism of microRNA396 mediating pistil development in Arabidopsis. Plant Physiol. 2014;164:249–58.
Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–82.
Harkess A, Zhou J, Xu C, Bowers JE, Van der Hulst R, Ayyampalayam S. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat Commun. 2017;8:1279.
Harkess A, Huang K, Van der Hulst R, Tissen B, Caplan JL, Koppula A, Batish M, Meyers BC, Leebens-Mackb J. Sex determination by two Y-linked genes in garden asparagus. Plant Cell. 2020;32:1790–6.
Manzanares C, Barth S, Thorogood D, Thorogood D, Byrne SL, Yates S, Czaban A, Asp T, Yang B, Studer B. A gene encoding a DUF247 domain protein cosegregates with the S self-Incompatibility locus in perennial ryegrass. Molr Biol Evol. 2016;33(4):870–84.
Thorogood D, Yates S, Manzanares C, Skot L, Hegarty M, Blackmore T, Barth S, Studer B. A novel multivariate approach to phenoty** and association map** of multi-locus gametophytic self-incompatibility reveals S, Z, and other loci in a perennial ryegrass (Poaceae) population. Front Plant Sci. 2017;8:1331.
Valleau WD. Inheritance of sex in the grape. Am Nat. 1915;597:554–64.
Fu JM, Liu HM, Hu JJ, Liang YQ, Liang JJ, Wuyun TN, Tan XF. Five complete chloroplast genome sequences from Diospyros: genome organization and comparative analysis. PLoS ONE. 2017;11(7):e0159566.
Suo YJ, Sun P, Cheng HH, Han WJ, Diao SF, Li HW, Mai YN, Zhao X, Li FD, Fu JM. A high-quality chromosomal genome assembly of Diospyros oleifera Cheng. GigaScience. 2020;9:1–10.
Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 1000 genome project data processing subgroup. The sequence alignment/map (SAM) format and SAMtools. Bioinformatics. 2009;25(6):2078–9.
Li R, Zhu H, Ruan J, Qian W, Fang X, Shi Z, Li Y, Li S, Shan G, Kristiansen K, Li S, Yang H, Wang J, Wang J. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20:265–72.
Sun P, Fu JM. The Diospyros oleifera heterozygous and male unmapped sequences. figshare. Dataset. 2022b. https://doi.org/10.6084/m9.figshare.20407386.v1.
Slifer SH. PLINK: Key Functions for data analysis. Curr Protoc Hum Genet. 2018;97:59.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9.
Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4.
Endelman JB. Ridge regression and other kernels for genomic selection with R package rrBLUP. Plant Genome-US. 2011;4(3):250–5.
Shin J, Blay S, McNeney B, Graham J. LDheatmap: An R function for graphical display of pairwise linkage disequilibria between single nucleotide polymorphisms. J Stat Softw. 2006;16:c03.
Mai Y, Diao S, Yuan J, Wang L, Suo Y, Li H, Han W, Wang Y, Ye L, Liu Y, et al. Identification and analysis of MADS-box, WRKY, NAC, and SBP-box transcription factor families in Diospyros oleifera Cheng and their associations with sex differentiation. Agronomy-basel. 2022;12:2100.
Acknowledgements
We thank Novogene Bioinformatics Institute for the technical assistance.
Funding
This study was supported by the National Key R&D Program of China (2018YFD1000606 and 2019YFD1000600) to JF and PS, the National Natural Science Foundation of China (32071801), and the Fundamental Research Funds for the Central Non-profit Research Institution of CAF (CAFYBB2017ZA005) to PS, and JSPS KAKENHI (21KK0269) to SN and (20K20454) to SN and RT.
Author information
Authors and Affiliations
Contributions
PS, SN, HL and YM contributed equally to this study. PS, SN, RT, FL and JF designed the study; PS, HL, YM and WH surveyed and collected the plant materials; PS, SN, HL and YM performed the experiments and analyzed the data with help from YS, SD, YW, JY and YZ; CL and HD assembled the genome; PS and SN drafted the manuscript, RT, JF and CL revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, P., Nishiyama, S., Li, H. et al. Genetic insights into the dissolution of dioecy in diploid persimmon Diospyros oleifera Cheng. BMC Plant Biol 23, 606 (2023). https://doi.org/10.1186/s12870-023-04610-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04610-3