Abstract
Background
Jasmonate ZIM-domain (JAZ) proteins, which act as negative regulators in the jasmonic acid (JA) signalling pathway, have significant implications for plant development and response to abiotic stress.
Results
Through a comprehensive genome-wide analysis, a total of 20 members of the JAZ gene family specific to alfalfa were identified in its genome. Phylogenetic analysis divided these 20 MsJAZ genes into five subgroups. Gene structure analysis, protein motif analysis, and 3D protein structure analysis revealed that alfalfa JAZ genes in the same evolutionary branch share similar exon‒intron, motif, and 3D structure compositions. Eight segmental duplication events were identified among these 20 MsJAZ genes through collinearity analysis. Among the 32 chromosomes of the autotetraploid cultivated alfalfa, there were 20 MsJAZ genes distributed on 17 chromosomes. Extensive stress-related cis-acting elements were detected in the upstream sequences of MsJAZ genes, suggesting that their response to stress has an underlying function. Furthermore, the expression levels of MsJAZ genes were examined across various tissues and under the influence of salt stress conditions, revealing tissue-specific expression and regulation by salt stress. Through RT‒qPCR experiments, it was discovered that the relative expression levels of these six MsJAZ genes increased under salt stress.
Conclusions
In summary, our study represents the first comprehensive identification and analysis of the JAZ gene family in alfalfa. These results provide important information for exploring the mechanism of JAZ genes in alfalfa salt tolerance and identifying candidate genes for improving the salt tolerance of autotetraploid cultivated alfalfa via genetic engineering in the future.
Similar content being viewed by others
Background
Alfalfa (Medicago sativa subsp. sativa), which is hailed as the “queen of forage” because of its ease of harvesting, digestibility by livestock, high protein content, and high yield, is a crop cultivated globally and covers an extensive planting area of approximately 30 million hectares, with an annual production reaching approximately 450 million tons [1]. Alfalfa is widely grown in the United States, Netherlands, and other countries, and it is the fourth most grown crop in the United States, with an annual cultivation area of 8.5–9.3 million hectares [2]. In China, alfalfa is mainly planted in northern regions, including provinces such as Gansu and ** method of JAZ proteins in Arabidopsis, all JAZ proteins on the evolutionary tree were categorized into groups. The gene structures of all MsJAZ genes were analysed using the Gene Structure Display Server (GSDS) (http://gsds.cbi.pku.edu.cn/). In addition, conserved motifs of all MsJAZ proteins were identified and analysed via the MEME 5.5.3 online tool (https://meme-suite.org/meme/), with a minimum motif length of 20, a maximum motif length of 100, a maximum number of motifs of 10, and a repetition number of 0 or 1 [42].
3D structure analysis of MsJAZ proteins
The 3D structure of a protein is the basis for its functional role. The secondary structures of all MsJAZ proteins, including α-helices, extended strands, β-turns, and random coils, were predicted and analysed using SOPMA.(https://npsa.lyon.inserm.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html) The quality of the predicted models was assessed using the Global Model Quality Estimation (GMQE) value.
Gene duplication analysis and chromosomal map**
TBtools software was used to identify duplication events in the 20 MsJAZ genes through collinearity analysis [S1,S2 and S3). The gene structures of all MsJAZ genes were analysed by GSDS, which showed that the number of coding sequences (exons) of the MsJAZ genes ranged from 3 to 8, among which MsJAZ3 had the largest number of exons (n = 8), while MsJAZ6, MsJAZ11, and MsJAZ12 had the smallest number of exons (n = 3) (Fig. 3B). Similarly, the number of exons in MsJAZ genes belonging to the same subgroup was similar. We used MEME to identify conserved motifs in MsJAZ proteins and their distribution, and detailed information on all motifs is shown in Table S5. JAZ proteins clustered in the same subgroup had motifs with similar numbers and types, indicating that they had similar functions (Fig. 3C). In addition, all MsJAZ proteins possessed motif 1 and motif 2.
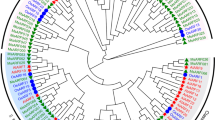
Phylogenetic relationships among JAZ proteins in alfalfa, Arabidopsis, M. truncatula, and rice. The phylogenetic tree was created by the NJ method. Different groups are indicated by different colours, and stars of different colours represent alfalfa, Arabidopsis, M. truncatula, and rice JAZ proteins
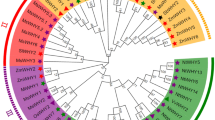
Phylogenetic relationships, gene structures, and conserved motifs. (A) Phylogenetic relationships of MsJAZ genes. The phylogenetic tree was created by the NJ method. (B) Gene structures of MsJAZ genes. The green boxes and black lines represent CDSs and introns, respectively. (C) Motif patterns of MsJAZ proteins. Boxes of different colours represent different motifs
Secondary structure and 3D structure analyses of MsJAZ proteins
The physical structures of proteins play important roles in their physiological and biochemical functions. Therefore, we predicted and analysed the secondary structures and 3D structures of all the MsJAZ proteins. The secondary structures of the MsJAZ proteins were determined by SOPMA, and the results showed that among all the MsJAZ proteins, those containing random coils comprised the largest proportion (51.38–72.19%), followed by those containing α-helices (10.66–28.91%), extended strands (5.15–18.49%), and β-turns (1.82–4.88%) (Table S6). SWISS-MODEL was used to predict the 3D structures of these proteins, and the GMQE value was used to evaluate the quality of these models (Fig. 4). The GMQE values of all the models were > 0.46, showing that these predicted 3D structures were reliable. These 3D structures were mainly composed of random coils and α-helices, which was consistent with the findings of the secondary structure analysis. Random coils existed in all the JAZ polypeptide chains and were the most widely distributed structural elements in the JAZ polypeptide chains. In addition, the 3D structures of the MsJAZ proteins on closely related evolutionary branches were similar, suggesting that these proteins may share similar physiological functions.
Synteny analysis and chromosomal map**
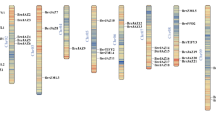
To identify gene duplication events, we performed collinearity analysis among these 20 MsJAZ genes. Gene replication events include segmental duplication, which refers to replication events between chromosomes, and tandem duplication, which refers to replication events within chromosomes. In our study, 8 pairs of segmental duplications in MsJAZ genes (MsJAZ1/MsJAZ2, MsJAZ5/MsJAZ9, MsJAZ5/MsJAZ10, MsJAZ9/MsJAZ10, MsJAZ7/MsJAZ19, MsJAZ12/MsJAZ13, MsJAZ15/MsJAZ16, and MsJAZ17/MsJAZ18) were identified, but no tandem duplications were detected (Fig. 5A). To understand the evolutionary constraints of JAZ gene duplication in alfalfa, the Ka, Ks, and Ka/Ks ratios for each segmental duplicated JAZ gene pair were analysed (Fig. 5B and Table S7). The Ka/Ks ratio of MsJAZ1/MsJAZ2 was greater than one (Ka/Ks = 3.201219), and those of the others were less than one, suggesting that purifying selection was the primary factor affecting the evolution of alfalfa JAZ genes. To investigate the role of JAZ genes in the direct evolution of different species, three comparative synteny maps were generated using TBtools software. These maps included comparisons between alfalfa and three reference species: Arabidopsis thaliana, Medicago truncatula, and Oryza sativa (Fig. 5C and Table S8). There were 12, 21, and 4 syntenic gene pairs between alfalfa and the three reference species Arabidopsis thaliana, Medicago truncatula, and Oryza sativa, respectively. Moreover, MsJAZ8 and MsJAZ17 had homologous gene pairs among these three species. Additionally, we analysed the chromosomal distribution of 20 MsJAZ genes in autotetraploid cultivated alfalfa, as shown in Fig. 6. The results revealed that the MsJAZ genes were distributed on various chromosomes of alfalfa. Specifically, there was only one MsJAZ gene present on chromosomes Chr1.2, Chr1.3, Chr1.4, Chr2.2, Chr2.4, Chr4.4, Chr5.2, Chr5.3, Chr5.4, Chr6.1, Chr6.3, Chr8.1, Chr8.2, Chr8.3, and Chr8.4. However, on chromosome Chr2.1, there were two MsJAZ genes (MsJAZ4 and MsJAZ5), and on chromosome Chr2.3, there were three MsJAZ genes (MsJAZ7, MsJAZ8, and MsJAZ9).
Synteny analysis of JAZ genes. (A) Synteny analysis of JAZ genes in the alfalfa genome. The grey lines in the central background represent all gene duplication events in the alfalfa genome. The red lines in the centre represent duplication events of JAZ genes. The outer circles in blue and red represent gene density. (B) Ka/Ks ratios of duplicated pairs of MsJAZ genes. The blue line indicates Ka/Ks = 1. (C) Synteny analysis of JAZ genes between alfalfa and Arabidopsis, Medicago truncatula, and rice. The grey lines in the background represent all gene duplication events. The red lines represent duplication events of JAZ genes
Analysis of promoter cis-acting elements
Identification and analysis of the cis-acting elements in the upstream sequences of genes allow us to determine whether these genes have the potential to participate in growth, development, and response to biotic or abiotic stresses. In this study, the cis-acting elements in the 2000 bp upstream regions of MsJAZ genes were identified and analysed via PlantCARE (Fig. 7). Many cis-acting elements were identified in these sequences, and based on their functions, they were classified into five categories: cell cycle regulation, plant development, hormone responses, stress responses, and transcription regulation. There were 2, 3, 12, 26, and 3 cis-acting elements associated with the cell cycle, development, hormones, stress, and transcription, respectively. A number of cis-regulatory elements, including AE-boxes, GATA motifs, GT1 motifs, LTRs, MBSs, and TC-rich repeats, have been demonstrated to be involved in the response to various abiotic and biotic stresses. In our study, we found that 11, 9, 15, 7, 8, and 7 MsJAZ genes contained AE-boxes, GATA motifs, GT1 motifs, LTRs, MBSs, and TC-rich repeats, respectively. In addition, all MsJAZ genes contained multiple CAAT-box and TATA-box cis-acting elements, indicating their potential significance in transcriptional regulation.
Expression pattern analysis of MsJAZ genes in different tissues
The expression of JAZ genes in various tissues of alfalfa reflects their specific functions in those tissues, providing the basis for the important role that JAZ genes play in the growth of alfalfa. Consequently, the expression levels of the 20 MsJAZ genes were determined across six different tissues, namely, leaves, flowers, pre-elongated stems, elongated stems, roots, and nodules. TBtools software was used to visualize the expression patterns of the MsJAZ genes, and the results showed that the expression profiles of the MsJAZ genes could be classified into three types (Fig. 8A). The expression of MsJAZ5, MsJAZ9, MsJAZ10, MsJAZ12, and MsJAZ20 was lowest in all tissues. MsJAZ1, MsJAZ2, MsJAZ4, MsJAZ7, MsJAZ8, MsJAZ15, MsJAZ16, MsJAZ17, and MsJAZ19 exhibited the highest expression levels in all tissues, indicating their significant role across various tissues. MsJAZ3, MsJAZ6, MsJAZ11, MsJAZ13, MsJAZ14, and MsJAZ18 exhibited high expression in some tissues and low expression in other tissues. Additionally, correlation analysis revealed that the expression patterns of most MsJAZ genes were positively correlated, but only a small subset of MsJAZ genes exhibited a significant direct correlation (Fig. 8B).
Expression analysis of alfalfa JAZ genes in six tissues. (A) Heatmap showing the differential expression of MsJAZ genes in six tissues. The values represent logarithmic conversions. Green and red indicate low and high MsJAZ gene expression, respectively. (B) Correlated heatmaps of the expression patterns in six tissues. The correlations are depicted using the colours blue and red, representing negative and positive correlations, respectively
Expression pattern analysis of MsJAZ genes in response to salt stress
To determine the dynamic changes in the gene expression of MsJAZ genes under salt stress treatment, we retrieved the expression levels of MsJAZ genes under salt stress through BLASTN. These transcriptome data were obtained after continuous salt treatment of alfalfa root tips for 0, 1, 3, 6, 12, and 24 h, as well as at 1 and 12 h after stress removal. Seventeen MsJAZ genes were found in this transcriptome dataset, and the remaining three genes were not found. TBtools software was used to construct a heatmap of the expression patterns of these 17 MsJAZ genes (Fig. 9A), and the R software package Mfuzz was used to analyse the time-related dynamic expression patterns of the MsJAZ genes (Fig. 9B). The expression trends of most of the MsJAZ genes were similar; with increasing stress duration, the expression levels increased and then decreased, and after the stress was removed, the levels immediately increased and then decreased, but the fold increase and response time differed. In addition, three clusters were formed by analysing the expression patterns of these 17 MsJAZ genes. The number of MsJAZ genes in Cluster 3 was the greatest (n = 8), followed by Cluster 2 (n = 6), and Cluster 1 contained the fewest (n = 3). The response patterns of the genes in Cluster 2 (MsJAZ1, MsJAZ4, MsJAZ7, MsJAZ14, MsJAZ17, and MsJAZ18) to salt stress were similar, and their expression levels were close and significantly greater than those of other MsJAZ genes. The genes in Cluster 1 (MsJAZ10, MsJAZ12, and MsJAZ16) had similar expression profiles, and their expression levels were close and significantly lower than those of the other clusters. The expression levels of the genes in Cluster 3 (MsJAZ2, MsJAZ5, MsJAZ8, MsJAZ9, MsJAZ13, MsJAZ15, MsJAZ19, and MsJAZ20) were moderate, and the changes were not significant.
Expression pattern analysis of alfalfa JAZ genes under continuous salt treatment. (A) The heatmap shows that the expression levels of the MsJAZ gene in the root tips changed at 0 (CK), 1, 3, 6, 12, and 24 h, as well as at 1 and 12 h after continuous salt treatment. The values in the figure are logarithmic conversions. Blue and red indicate low and high MsJAZ gene expression, respectively. (B) Expression trend analysis of MsJAZ genes at 0 (CK), 1, 3, 6, 12, and 24 h of salt treatment and at 1 and 12 h after continuous salt treatment
RT‒qPCR validation
To further study the response of the MsJAZ gene to salt stress, we selected six MsJAZ genes (MsJAZ1, MsJAZ4, MsJAZ7, MsJAZ14, MsJAZ17, and MsJAZ18) whose expression was positively induced by salt stress for RT‒qPCR experiments, and the results are shown in Fig. 10A. The expression patterns of all the selected MsJAZ genes were found to be consistent with the RNA-Seq data, indicating that the expression of these genes increased under salt stress. Among these genes, the expression of MsJAZ1 continued to increase and peaked at 1 h after removal of salt treatment, which was in line with the RNA-Seq data. The expression trends of the other five genes were similar; they all increased with increasing salt stress, decreased after reaching the peak value, and then peaked a second time at 1 h after salt removal, but they first peaked at different times. Overall, the expression trend of all MsJAZ genes in the RT‒qPCR assay was in line with the RNA‒Seq results, but the magnitudes of the changes in the RNA‒Seq and RT‒qPCR results differed. In addition, except for MsJAZ1/MsJAZ14 and MsJAZ7/MsJAZ14, which were negatively correlated, the other genes were directly positively correlated (Fig. 10B).
Relative expression levels of six selected MsJAZ genes under salt treatment. (A) The relative expression levels of six MsJAZ genes under salt treatment for different durations determined by RT‒qPCR. The data in the figure are the means ± SDs of three replicates. Asterisks indicate significant differences, and p < 0.05 (*) indicates extreme significance. (B) The correlation of the gene expression patterns of the six MsJAZ genes. Black and yellow indicate positive and negative correlations, respectively
Subcellular localization of the MsJAZ1 protein
The subcellular localization prediction of the selected MsJAZ1 protein by WoLF PSORT revealed its localization in the nucleus (Table S3). To validate this prediction and further investigate the genes significantly induced by salt stress, we performed transient expression experiments in tobacco leaves. The results showed that the GFP fluorescence overlapped with DAPI staining, indicating its localization in the nucleus, consistent with the predicted results (Fig. 11).
Discussion
Jasmonoyl-L-isoleucine (JA-Ile) is a crucial signalling molecule that plays a significant role in regulating various aspects of plant growth, development, and defence responses [23, 50, 51]. JAZ proteins are key components of the JA pathway and can negatively regulate signal transduction of JAs. JAZ proteins inhibit the activity of DNA-binding transcription factors that regulate the transcription of JA response genes [36]. To improve the adaptability of plants to adverse conditions, plants need to inhibit growth and other vital activities, and the expression of the JAZ gene can reduce the production of JA, which can regulate metabolic processes [35]. In addition, with the continuous development of biotechnology, the genome-wide identification of JAZ genes has been performed for several plants, such as Arabidopsis [25, 30], rice [52], maize [53], Sorghum bicolor [38], common Fig. [54], bread wheat [55], tomato [56], tea [57], and soybean [58]. However, no comprehensive identification or analysis of the alfalfa JAZ gene family has been reported.
Here, 20 JAZ genes were identified in the **nJiangDaYe genome. The number of JAZ genes in alfalfa was greater than that in Arabidopsis (n = 13) [25, 30], rice (n = 15) [52], maize (n = 16) [53], Sorghum bicolor (n = 17) [38], common fig (n = 10) [54], bread wheat (n = 14) [55], tomato (n = 9) [56], and tea (n = 13) [57] but less than that in soybean (n = 33) [58]. The MsJAZ proteins differed in length, molecular weight (MW), and theoretical isoelectric point (PI) but exhibited similar instability indices (IIs) and grand average hydropathicity indices (GRAVYs). Through subcellular localization prediction, we found that all the MsJAZ proteins were located in the nucleus, while the MsJAZ5, MsJAZ9, and MsJAZ10 proteins may also be located in the cell membrane. The subcellular localization experiment of MsJAZ1 protein was conducted, and the results were consistent with the prediction. The protein properties and subcellular localization predictions of MsJAZ proteins were similar to those in other plants [31, 39]. According to the GO analysis results, all MsJAZ genes were associated with biological processes and molecular functions, indicating that they are important for the growth and development of alfalfa.
Phylogenetic analysis revealed that the 20 MsJAZ genes could be divided into five subfamilies based on their evolutionary relationships with Arabidopsis JAZ genes. The results obtained via the NJ, ML, ME, and UPGMA methods were similar, but those obtained via the NJ method were more similar to those of the Arabidopsis JAZ gene classification [25, 30]. The clustering of JAZ genes from alfalfa and M. truncatula in the same branch was the most prominent, indicating that the genetic relationship of alfalfa with M. truncatula was closer than that with Arabidopsis or rice. Genes located on the same evolutionary branch may have the same function, so the same function of these genes can be predicted based on homology and phylogenetic analysis with other species [59]. AtJAZ1 [60] and OsJAZ9 [33] have been demonstrated to be associated with the regulation of salt stress, and thus, the MsJAZ proteins with AtJAZ1 and OsJAZ9 in group I may regulate salt stress. All MsJAZ genes contained three to eight exons and two to eight motifs, and the MsJAZ genes in the same subfamily had similar gene structures and motifs, indicating that these genes in the same subgroup may have subgroup-specific functions. All MsJAZ genes had motif 1 and motif 2. Motif 1 and motif 2 were identified as the TIFY domain and JAS domain, respectively, according to the NCBI-CDD results, and their positions were consistent with those in previous studies [26, 30]. Many of the 3D structures of MsJAZ proteins are similar, and therefore, MsJAZ proteins may have similar functions [61].
Gene replication plays a very important role in the evolutionary expansion of all gene families in plants [62]. In our study, 8 pairs of segmental duplications but no tandem duplications were detected in alfalfa, indicating that segmental duplications rather than tandem duplications played an important role in the evolutionary expansion of MsJAZ. Most Ka/Ks ratios of duplicated gene pairs were less than one, suggesting that the JAZ genes mainly evolved under the effect of purifying selection in alfalfa [63]. Identification of homologous genes between different species by collinearity analysis is helpful for understanding the role of genes in plant evolution [64]. Therefore, we analysed and compared the homologous genes of alfalfa with those of A. thaliana, M. truncatula, and O. sativa. The results showed that there were more homologous gene pairs between alfalfa and M. truncatula than between A. thaliana and O. sativa, suggesting that the genetic background of autotetraploid alfalfa is more similar to that of M. truncatula. A similar phenomenon has been found in cassava [65]. In addition, we found that MsJAZ8 and MsJAZ17 were collinear in all the plants, indicating that these genes played a vital role in the evolution of JAZ genes.
Cis-acting element analysis can help predict the possible roles of genes in biotic and abiotic stress signal responses [66]. Many stress-related cis-acting elements, such as ABREs, anaerobic induction essential elements (AREs), low-temperature response elements (LTRs), and MYB-binding sites involved in drought responses (MBSs), have been identified in the promoter sequences of MsJAZ genes, and these elements were also found in the JAZ gene family members of sweet potato [37]. These cis-acting elements have been reported to be associated with stress. For example, ABREs can interact with upstream transcription factors to activate ABA-responsive genes, thereby improving plant stress resistance [67]. In addition, these stress-related cis-acting elements were also present in the promoter regions of stress response genes, such as MsDof [68], MsWRKY [69], and MsMYB [70]. The MsJAZ genes had these cis-acting elements, suggesting that they have the potential to respond to salt stress.
JA can promote plant senescence and inhibit seedling growth, while the JAZ protein, an important member of the JA signal transduction pathway, also plays an important role in plant growth and development [10, 11]. In Arabidopsis, JAZ4 functions as a negative regulator of ethylene (ET) signalling and auxin signalling in the root tissues above the apex, but in the root apex, JAZ4 might act as a positive regulator of auxin signalling, potentially independent of ethylene signalling, and these distinct roles have an impact on root growth and development [71]. In soybeans, GmJAZ3 interacts with GmRR18a and GmMYC2a to regulate seed size and weight [72]. In different tissues, the regulatory function of genes mainly depends on their expression levels [73]. In our study, the MsJAZ genes were differentially expressed in different tissues, suggesting differences in their functions in plant growth and development. MsJAZ1, MsJAZ2, MsJAZ4, MsJAZ7, MsJAZ8, MsJAZ15, MsJAZ16, MsJAZ17, and MsJAZ19 were significantly more highly expressed than other MsJAZ genes in all tissues, indicating that these genes may be important at all stages of plant development. Moreover, MsJAZ13 and MsJAZ14 were highly expressed in leaves, which indicated that these MsJAZ genes could control leaf development.
JA is essential for the growth-defence balance of plants, as JA can both promote defence and inhibit plant growth [74]. The related role of JAZ genes in salt tolerance has been reported in many plants. For instance, GhJAZ1 can promote the expression of resistance-related genes and root growth, increasing the vitality, height, fresh weight, and fruiting rate of transgenic plants, thereby enhancing the activity of upland cotton (Gossypium hirsutum) plants under salt stress [75]. Overexpression of apple MdJAZ2 enhanced the salt tolerance of Arabidopsis [76]. PnJAZ1, through crosstalking with the abscisic acid signalling pathway, enhances the growth of Pohlia nutans plants under salt stress [77]. In this study, a total of 17 MsJAZ genes were identified based on our RNA-Seq data. Overall, the expression levels of the majority of these MsJAZ genes were upregulated under salt treatment. These findings suggested that most MsJAZ genes likely play a positive regulatory role in the response to salt stress. Additionally, we performed RT‒qPCR experiments on six genes that were found to be significantly induced by salt stress according to the RNA-Seq analysis. We observed a significant increase in their relative expression levels under salt stress, which was consistent with the expression trends observed in the RNA-Seq analysis. However, the fold change in the expression levels of these selected genes differed between the RNA-Seq analysis and the RT‒qPCR experiment. This discrepancy may be attributed to the biological diversity among different individuals of alfalfa [78].
Conclusions
In this study, a total of 20 JAZ gene family members were identified in the autotetraploid cultivated alfalfa genome. Several aspects of these genes were investigated, including their physicochemical properties, evolutionary relationships, gene structures, protein motif compositions, 3D protein structures, gene duplication events, chromosomal distribution, and cis-acting elements, as well as their expression levels in different tissues and under salt stress conditions. In addition, expression analysis revealed that MsJAZ1, MsJAZ4, MsJAZ7, MsJAZ14, MsJAZ7 and MsJAZ18 significantly responded to salt stress. In conclusion, our study is the first to provide a comprehensive identification and analysis of alfalfa JAZ gene family members at the autotetraploid level. These findings establish a strong foundation for future research on the function and molecular mechanisms of JAZ genes in the salt stress response of autotetraploid cultivated alfalfa.
Data availability
The draft genome data of autotetraploid cultivated alfalfa was obtained from (figshare.com/projects/whole_genome_sequencing_and_assembly_of_Medicago_sativa/66380). Genome-wide transcriptome data of different alfalfa tissues were acquired from the MODMS (modms.lzu.edu.cn). All transcriptome sequencing data used in this study were available in NCBI SRA (www.ncbi.nlm.nih.gov/sra/): SRR7160313-SRR7160357 (salt treatment).
Abbreviations
- JAZ:
-
Jasmonate-ZIM
- HMM:
-
Hidden Markov Model
- GO:
-
Gene Ontology
- JA:
-
Jasmonic Acid
- JA-Ile:
-
Jasmonoyl-Isoleucine
- Jas:
-
Jasmonates
- MeJA:
-
Methyl Jasmonate
- Chr:
-
Chromosome
References
Barros J, Templet S, Dixon RA. Development and commercialization of reduced lignin alfalfa. Curr Opin Biotech. 2019;56:48–54.
Chen HT, Zeng Y, Yang YZ, Huang LL, Tang BL, Zhang H, Hao F, Liu W, Li YH, Liu YB, Zhang XS, Zhang R, Zhang YS, Li YX, Wang K, He H, Wang ZK, Fan GY, Yang H, Bao AK, Shang ZH, Chen JH, Wang W, Qiu Q. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat Commun. 2020;11:2494.
Jia QM, Kamran M, Ali S, Sun LF, Zhang P, Ren XL, Jia ZK. Deficit irrigation and fertilization strategies to improve soil quality and alfalfa yield in arid and semi-arid areas of northern China. Peer J. 2018;6:e4410.
Bao AK, Du BQ, Touil L, Kang P, Wang QL, Wang SM. Co-expression of tonoplast Cation/H + antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylum improves alfalfa plant growth under salinity, drought and field conditions. Plant Biotechnol J. 2016;14(3):964–75.
Ouhibi C, Attia H, Rebah F, Msilini N, Chebbi M, Aarrouf J, Urban L, Lachaal M. Salt stress mitigation by seed priming with UV-C in lettuce plants: growth, antioxidant activity and phenolic compounds. Plant Physiol Biochem. 2014;83:126–33.
Zhang HL, Yu FF, **e P, Sun SY, Qiao XH, Tang SY, Chen CX, Yang S, Mei C, Yang DK, Wu YR, **a R, Li X, Lu J, Liu YX, **e XW, Ma DM, Xu X, Liang ZW, Feng ZH, Huang XH, Yu H, Liu GF, Wang YC, Li JY, Zhang QF, Chen C, Ouyang YD, **e Q. A Gγ protein regulates alkaline sensitivity in crops. Science. 2023;379(6638):eade8416.
Ismail A, Takeda S, Nick P. Life and death under salt stress: same players, different timing? J Exp Bot. 2014;65(12):2963–79.
Slama I, Abdelly C, Bouchereau A, Flowers T, Savoure A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot. 2015;115(3):433–47.
Wani SH, Kumar V, Shriram V, Sah SK. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016;4(3):162–76.
Ueda J, Kato J. Isolation and identification of a senescence-promoting substance from wormwood (Artemisia absinthium L). Plant Physiol. 1980;66(2):246–9.
Dathe W, RÖnsch H, Preiss A, Schade W, Sembdner G, Schreiber K. Endogenous plant hormones of the broad bean, Vicia faba L. (-)-jasmonic acid, a plant growth inhibitor in pericarp. Planta. 1981;153:530–5.
Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2015;43:205–27.
Song SS, Huang H, Gao H, Wang JJ, Wu DW, Liu XL, Yang SH, Zhai QZ, Li CY, Qi TC, **e DX. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell. 2014;26(1):263–79.
Yan YX, Christensen S, Isakeit T, Engelberth J. Disruption of OPR7 and OPR8 reveals the versatile functions of Jasmonic Acid in Maize Development and Defense. Plant Cell. 2012;24(4):1420–36.
Ye M, Luo SM, **e JF, Li YF. Silencing COI1 in rice increases susceptibility to chewing insects and impairs inducible defense. PLoS ONE. 2012;7:e36214.
Hudgins JW, Christiansen E, Franceschi VR. Induction of anatomically based defense responses in stems of diverse conifers by methyl jasmonate: a phylogenetic perspective. Tree Physiol. 2004;24(3):251–64.
Schmelz EA, Huffaker A, Sims JW, Christensen SA, Lu X, Okada K, Peters RJ. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014;79(4):659–78.
Zulak KG, Bohlmann J. Terpenoid biosynthesis and specialized vascular cells of conifer defense. J Integr Plant Biol. 2010;52(1):86–97.
Yan J, Li H, Li S, Yao R, Deng H, **e Q, **e D. The Arabidopsis F-box protein CORONATINE INSENSITIVE1 is stabilized by SCFCOI1 and degraded via the 26S proteasome pathway. Plant Cell. 2013;25(2):486–98.
Farmer EE, Johnson RR, Ryan CA. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol. 1992;98(3):995–1002.
Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87(19):7713–6.
Abouelsaad I, Renault S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J Plant Physiol. 2018;226:136–44.
Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in annals of Botany. Ann Bot. 2013;111(6):1021–58.
Ma PD, Pei TL, Lv BB, Wang M, Dong JN, Liang ZS. Functional pleiotropism, diversity, and redundancy of Salvia miltiorrhiza Bunge JAZ family proteins in jasmonate-induced tanshinone and phenolic acid biosynthesis. Hortic Res. 2022;9:uhac166.
Bai Y, Meng Y, Huang D, Qi Y, Chen M. Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics. 2011;98(2):128–36.
Ju L, **g Y, Shi P, Liu J, Chen J, Yan J, Chu J, Chen KM, Sun J. JAZ proteins modulate seed germination through interaction with ABI5 in bread wheat and Arabidopsis. New Phytol. 2019;223(1):246–60.
Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Perez AC, Chico JM, Vanden Bossche R, Sewell J, Gil E, García-Casado G, Witters E, Inzé D, Long JA, De Jaeger G, Solano R, Goossens A. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature. 2010;464:788–91.
Shyu C, Figueroa P, Depew CL, Cooke TF, Sheard LB, Moreno JE, Katsir L, Zheng N, Browse J, Howe GA. JAZ8 lacks a canonical degron and has an EAR motif that mediates transcriptional repression of jasmonate responses in Arabidopsis. Plant Cell. 2012;24(2):536–50.
Chung HS, Howe GA. A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell. 2009;21(1):131–45.
Thireault C, Shyu C, Yoshida Y, St Aubin B, Campos ML, Howe GA. Repression of jasmonate signaling by a non-TIFY JAZ protein in Arabidopsis. Plant J. 2015;82(4):669–79.
Song HY, Duan ZH, Wang Z, Li Y, Wang YY, Li CM, Mao WM, Que QM, Chen XY, Li P. Genome-wide identification, expression pattern and subcellular localization analysis of the JAZ gene family in Toona ciliata. Ind Crop Prod. 2022;178:114582.
Sun H, Chen L, Li JY, Hu ML, Ullah A, He X, Yang XY, Zhang XL. The JASMONATE ZIM-Domain Gene Family mediates JA signaling and stress response in cotton. Plant Cell Physiol. 2017;58(12):2139–54.
**ong LZ, Zhang T, Wu H, Ye HY, Yao RF. OsJAZ9 acts as a transcriptional regulator in jasmonate signaling and modulates salt stress tolerance in rice. Plant Sci. 2015;232:1–12.
Zhu D, Cai H, Luo X, Bai X, Deyholos MK, Chen Q, Chen C, Ji W, Zhu Y. Over-expression of a novel JAZ family gene from Glycine soja, increases salt and alkali stress tolerance. Biochem Biophys Res Commun. 2012;426(2):273–9.
Bhanbhro N, **ao BB, Han L, Lu HY, Wang H, Yang CW. Adaptive strategy of allohexaploid wheat to long-term salinity stress. BMC Plant Biol. 2020;20:210.
Wasternack C, Song S. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J Exp Bot. 2017;68(6):1303–21.
Huang ZW, Wang Z, Li X, He SZ, Liu QC, Zhai H, Zhao N, Gao SP, Zhang H. Genome-wide identification and expression analysis of JAZ family involved in hormone and abiotic stress in sweet potato and its two diploid relatives. Int J Mol Sci. 2021;22(18):9786.
Du QL, Fang YP, Jiang JM, Chen MQ, Li XY, **e X. Genome-wide identification and characterization of the JAZ gene family and its expression patterns under various abiotic stresses in Sorghum bicolor. J Integr Agr. 2022;21(12):3540–55.
Jia K, Yan CY, Zhang J, Cheng YX, Li WW, Yan HZ, Gao J. Genome-wide identification and expression analysis of the JAZ gene family in turnip. Sci Rep. 2021;11:21330.
Luo D, Zhou Q, Wu YG, Chai XT, Liu WX, Wang YR, Yang QC, Wang ZY, Liu ZP. Full-length transcript sequencing and comparative transcriptomic analysis to evaluate the contribution of osmotic and ionic stress components towards salinity tolerance in the roots of cultivated alfalfa (Medicago sativa L). BMC Plant Biol. 2019;19:32.
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8.
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME suite: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–8.
Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, **a R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202.
Wang D, Zhang Y, Zhang Z, Zhu J, Yu J. KaKs_Calculator 2.0: a Toolkit incorporating Gamma-Series methods and sliding window strategies. Genom Proteom Bioinf. 2010;8(1):77–80.
Chao JT, Li ZY, Sun YH, Aluko OO, Wu XR, Wang Q, Liu GS. MG2C: a user-friendly online tool for drawing genetic maps. Mol Hortic. 2021;1:16.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Dong XM, Han BC, Yin XY, Mao P, Luo D, Zhou Q, Liu ZP. Genome-wide identification of the GRAS transcription factor family in autotetraploid cultivated alfalfa (Medicago sativa L.) and expression analysis under drought stress. Ind Crops Prod. 2023;194:116379.
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421.
Dong XM, Deng H, Ma WX, Zhou Q, Liu ZP. Genome-wide identification of the MADS-box transcription factor family in autotetraploid cultivated alfalfa (Medicago sativa L.) and expression analysis under abiotic stress. BMC Genom. 2021;22:603.
Howe G, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66.
Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol. 2009;60:183–205.
Ye HY, Du H, Tang N, Li XH, **ong LZ. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol Biol. 2009;71:291–305.
Han Y, Luthe D. Identification and evolution analysis of the JAZ gene family in maize. BMC Genom. 2021;22:256.
Song MY, Wang HM, Ma HQ, Zheng CL. Genome-wide analysis of JAZ family genes expression patterns during fig (Ficus carica L.) fruit development and in response to hormone treatment. BMC Genom. 2022;23:170.
Wang Y, Qiao L, Bai J, Wang P, Duan W, Yuan S, Yuan G, Zhang F, Zhang L, Zhao C. Genome-wide characterization of JASMONATE-ZIM DOMAIN transcription repressors in wheat (Triticum aestivum L). BMC Genom. 2017;18:152.
Chini A, Ben-Romdhane W, Hassairi A, Aboul-Soud MAM. Identification of TIFY/JAZ family genes in Solanum lycopersicum and their regulation in response to abiotic stresses. PLoS ONE. 2017;12(6):e0177381.
Zheng Y, Chen X, Wang P, Sun Y, Yue C, Ye N. Genome-wide and expression pattern analysis of JAZ family involved in stress responses and postharvest processing treatments in Camellia sinensis. Sci Rep. 2020;10:2792.
Zhang BX, Zheng H, Wu HH, Wang CL, Liang ZS. Recent genome-wide replication promoted expansion and functional differentiation of the JAZs in soybeans. Int J Biol Macromol. 2023;238:124064.
Kapli P, Yang Z, Telford MJ. Phylogenetic tree building in the genomic age. Nat Rev Genet. 2020;21:428–44.
Luo X, Li C, He X, Zhang X, Zhu L. ABA signaling is negatively regulated by GbWRKY1 through JAZ1 and ABI1 to affect salt and drought tolerance. Plant Cell Rep. 2020;39:181–94.
Feng WX, Mehari TG, Fang H, Ji MJ, Qu ZJ, Jia MX, Wang DM, Ditta A, Khan MKR, Cao YY, Wu JY, Wang BH. Genome-wide identification of the geranylgeranyl pyrophosphate synthase (GGPS) gene family involved in chlorophyll synthesis in cotton. BMC Genom. 2023;24:176.
Fischer I, Diévart A, Droc G, Dufayard J, Chantret N. Evolutionary dynamics of the leucine-rich repeat receptor-like kinase (LRR-RLK) subfamily in Angiosperms. Plant Physiol. 2016;170(3):1595–610.
Qiang YQ, He XJ, Li Z, Li SQ, Zhang J, Liu T, Tursunniyaz M, Wang XY, Liu ZP, Fang LF. Genome-wide identification and expression analysis of the response regulator gene family in alfalfa (Medicago sativa L.) reveals their multifarious roles in stress response. Front Plant Sci. 2023;14:1149880.
Ray M, Robert VB, Jane SM. Genome of the long-living sacred lotus (Nelumbo nucifera Gaertn). Genome Biol. 2013;14:R41.
Yang TY, Li HR, Li LW, Wei WL, Huang YH, **ong FQ, Wei MG. Genome-wide characterization and expression analysis of α-amylase and β-amylase genes underlying drought tolerance in cassava. BMC Genom. 2023;24:190.
Ma YT, Wei N, Wang QX, Liu ZP, Liu WX. Genome-wide identification and characterization of the heavy metal ATPase (HMA) gene family in Medicago truncatula under copper stress. Int J Biol Macromol. 2021;193:893–902.
Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006;57:781–803.
Cao B, Cui Y, Lou KK, Luo D, Liu ZP, Zhou Q. Genome-wide identification and expression analysis of the Dof gene family in Medicago sativa L. under various abiotic stresses. DNA Cell Biol. 2020;39(11):1976–89.
Mao P, ** XY, Bao QY, Mei C, Zhou Q, Min XY, Liu ZP. WRKY transcription factors in Medicago sativa L.: genome-wide identification and expression analysis under abiotic stress. DNA Cell Biol. 2020;39(12):2212–25.
Zhou Q, Jia CL, Ma WX, Cui Y, ** XY, Luo D, Min XY, Liu ZP. MYB transcription factors in alfalfa (Medicago sativa): genome-wide identification and expression analysis under abiotic stresses. Peer J. 2019;7:e7714.
DeMott L, Oblessuc PR, Pierce A, Student J, Melotto M. Spatiotemporal regulation of JAZ4 expression and splicing contribute to ethylene- and auxin-mediated responses in Arabidopsis roots. Plant J. 2021;108(5):1266–82.
Hu Y, Liu Y, Tao JJ, Lu L, Jiang ZH, Wei JJ, Wu CM, Yin CC, Li W, Bi YD, Lai YC, Wei W, Zhang WK, Chen SY, Zhang JS. GmJAZ3 interacts with GmRR18a and GmMYC2a to regulate seed traits in soybean. J Integr Plant Biol. 2023;65(8):1983–2000.
Huang W, Carbone MA, Lyman RF, Anholt RRH, Mackay TFC. Genotype by environment interaction for gene expression in Drosophila melanogaster. Nat Commun. 2020;11:5451.
Major IT, Guo Q, Zhai JL, Kapali G, Kramer DM, Howe GA. A phytochrome B-independent pathway restricts growth at high levels of jasmonate defense. Plant Physiol. 2020;183(2):733–49.
Zhao G, Song Y, Wang QH, Yao DX, Li DL, Qin WQ, Ge XY, Yang ZR, Xu WY, Su Z, Zhang XY, Li FG, Wu JH. Gossypium hirsutum salt tolerance is enhanced by overexpression of G. Arboreum JAZ1. Front Bioeng Biotech. 2020;8:157.
An XH, Hao YJ, Li EM, Xu K, Cheng CG. Functional identification of apple MdJAZ2 in Arabidopsis with reduced JA-sensitivity and increased stress tolerance. Plan Cell Rep. 2017;36:255–65.
Liu S, Zhang P, Li C, **a G. The moss jasmonate ZIM-domain protein PnJAZ1 confers salinity tolerance via crosstalk with the abscisic acid signalling pathway. Plant Sci. 2019;280:1–11.
Zhou Q, Che TL, Wang YR, Liu ZP. The development of 204 novel EST-SSRs and their use for genetic diversity analyses in cultivated alfalfa. Biochem Syst Ecol. 2014;57:227–30.
Acknowledgements
Not applicable.
Funding
This research was supported by the Biological Breeding Project (2022ZD04011), the Inner Mongolia Seed Industry Science and Technology Innovation Major Demonstration Project (2022JBGS0040), the China Postdoctoral Science Foundation (2022M721442) and the Natural Science Foundation of Gansu Province (23JRRA1151).
Author information
Authors and Affiliations
Contributions
ZPL and WY conceived and designed the experiment. WY, XMD, RL, XLZ, QZ and DL performed the experiments. WY and XMD analyzed all the data. WY wrote the manuscript. ZPL revised the manuscript. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We conducted the experimental research on cultivated alfalfa in accordance with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora. Alfalfa seeds of **nJiangDaYe was provided by the National Livestock Husbandry Station, Ministry of Agriculture and Rural Affairs of The People’s Republic of China.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yan, W., Dong, X., Li, R. et al. Genome-wide identification of JAZ gene family members in autotetraploid cultivated alfalfa (Medicago sativa subsp. sativa) and expression analysis under salt stress. BMC Genomics 25, 636 (2024). https://doi.org/10.1186/s12864-024-10460-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10460-6