Abstract
Background
Geranylgeranyl pyrophosphate synthase (GGPS) is a structural enzyme of the terpene biosynthesis pathway that is involved in regulating plant photosynthesis, growth and development, but this gene family has not been systematically studied in cotton.
Results
In the current research, genome-wide identification was performed, and a total of 75 GGPS family members were found in four cotton species, Gossypium hirsutum, Gossypium barbadense, Gossypium arboreum and Gossypium raimondii. The GGPS genes were divided into three subgroups by evolutionary analysis. Subcellular localization prediction showed that they were mainly located in chloroplasts and plastids. The closely related GGPS contains a similar gene structure and conserved motif, but some genes are quite different, resulting in functional differentiation. Chromosome location analysis, collinearity and selection pressure analysis showed that many fragment duplication events occurred in GGPS genes. Three-dimensional structure analysis and conservative sequence analysis showed that the members of the GGPS family contained a large number of α-helices and random crimps, and all contained two aspartic acid-rich domains, DDxxxxD and DDxxD (x is an arbitrary amino acid), suggesting its key role in function. Cis-regulatory element analysis showed that cotton GGPS may be involved in light response, abiotic stress and other processes. A GGPS gene was silenced successfully by virus-induced gene silencing (VIGS), and it was found that the chlorophyll content in cotton leaves decreased significantly, suggesting that the gene plays an important role in plant photosynthesis.
Conclusions
In total, 75 genes were identified in four Gossypium species by a series of bioinformatics analysis. Gene silencing from GGPS members of G. hirsutum revealed that GGPS plays an important regulatory role in photosynthesis. This study provides a theoretical basis for the biological function of GGPS in cotton growth and development.
Similar content being viewed by others
Background
Geranylgeranyl pyrophosphate synthase (GGPS) is a structural enzyme in the terpene biosynthesis pathway and a member of the isopentenyl pyrophosphate synthase gene family. Terpenoids are the largest and most diverse plant-specific metabolites and play important biological roles in various physiological processes, such as growth, photosynthesis, signal transduction, environmental adaptation and stress tolerance, during plant development [1]. All terpenoids are derived from the basic unit structure of five carbon atoms: isopentenyl pyrophosphate (IPP) and its allyl isomer dimethyl allyl pyrophosphate (DMAPP). In the plastids of plants, IPP and DMAPP are synthesized by the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway. Three molecules of IPP and one molecule of DMAPP form the 20-carbon compound geranylgeranyl pyrophosphate (GGPP) in the action of GGPS. GGPP continues to be catalyzed to form diterpenes and tetri-terpenes [2]. GGPP is not only the precursor of diterpenoids and carotenoids but also the common precursor of tocopherol, abscisic acid, gibberellin, quinone and other polyterpenes. It is the node of many important secondary metabolic pathways in plants [3]. GGPS is a key enzyme in the synthesis of octahydrolycopene in carotenoids [4]. Carotenoid is a fat-soluble pigment that is often located in the chloroplast and chromoplast membranes. Carotenoids can protect chlorophyll from photooxidation damage caused by strong light, and they are an indispensable structural component of the photosynthetic antenna and reaction center complex. In addition, carotenoids are an important component of some pigment-protein complexes [5] and are the precursor of abscisic acid (ABA) [6]. The GGPS gene was first isolated from pepper [7] and then isolated from tomato, Salvia miltiorrhiza, tobacco, Ginkgo biloba and other plants. In relation to the role of glucose, the enzyme UDP-glucose pyrophosphorylase (UGP), a member of the glycosyltransferase gene family, catalyzes the reaction between glucose-1-phosphate and UTP to produce uridine diphosphate glucose (UDPG). Different roles are played by UGP genes. UGP is a necessary substance for b-1,3 glucan and b-1,6 glucan both of which are basic building blocks for the biosynthesis of the cell wall in fungi in the formation of UDPG [8].
In recent years, some progress has been made in the functional research of GGPS genes. The physiological and biochemical functions of GGPS are closely related to its tissue expression characteristics and subcellular localization. There are 12 GGPS genes in the Arabidopsis thaliana genome [9]. Different family members are responsible for the synthesis of GGPP in different subcellular structures, in which AtGGPS1 and AtGGPS3 are located in plastids, AtGGPS2 and AtGGPS4 are distributed in mitochondria, and AtGGPS6 is located in the endoplasmic reticulum [10]. GGPS1, located in mitochondria, uses GPP to synthesize gibberellins and GGPS11, which are located in plastids and are the core of photosynthesis. GGPP is widely used in the synthesis of chlorophylls, carotenoids and other compounds [11]. Two GGPS genes have been identified in tomato, and the two genes were found in all tissues and organs. In sweet potato, overexpression of the IbGGPS gene can upregulate genes related to the glycolysis pathway, MEP pathway and carotenoid pathway and increase the content of carotenoids in transgenic plants. These results suggested that the IbGGPS gene has the potential to increase the content of carotenoids in sweet potato and other plants [12]. In addition, LeGGPS1 expression can be induced when plants are subjected to biological stress [13]. The specifically expressed GGPS genes in flowers and fruits are involved in the synthesis of carotenoids, and the specifically expressed GGPS in leaves is involved in the synthesis of insect pest-induced volatiles (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT) [13]. In addition, terpenoids are induced under abiotic stresses, such as UV-B rays, gamma rays, high temperature or the production of reactive oxygen species (ROS) [14]. Under a high-temperature environment, Quercus ilex uses monoterpenes to scavenge free radicals, ROS, etc., and releases a large number of volatile monoterpenes to reduce tree body temperature [15]. GGPS is not only important for plant growth and development [16] but has also been widely reported in bacteria [17], fungi [18], insects [19] and animals [20].
Cotton is one of the main cash crops in the world [21]. GGPS is a crucial enzyme for the production of gibberellins, carotenoids, chlorophylls, and rubber, which are structurally diverse classes of isoprenoid biosynthetic metabolites produced by GGPP synthase (GGPPS) in plastids [22, 23]. Currently, the GGPS gene family has been identified in a variety of plants, but there is not enough research on this gene family and related biological function analysis in cotton. Therefore, analyzing the evolution and function of the GGPS gene family in cotton is helpful to screen excellent cotton germplasm resources and deepen the understanding of the biological function of the GGPS gene family. In this study, we downloaded the genome data of four cotton species, namely, Gossypium hirsutum, Gossypium arboreum, Gossypium raimondii and Gossypium barbadense, from the cotton database and revealed their evolutionary analysis, cis-acting elements, gene structure, conserved motifs, chromosome location, protein structure and other information through a series of bioinformatics methods. This study provides a theoretical reference for revealing the regulatory mechanism of genetic evolution, growth and chlorophyll synthesis of this gene family in cotton.
Results
Identification and sequence analysis of the GGPS gene family in cotton
Here, we identified 75 GGPS genes in four cotton species from the cottonFGD and Phytozome databases. There were 14, 12, 22 and 27 genes in G. raimondii, G. arboreum, G. barbadense and G. hirsutum, respectively. Then, the physiochemical properties and sequences of the members of the GGPS gene family were analyzed (Table S1). The protein molecular weights of GGPS genes were between 14,371.5 ~ 46,093.3 Da, and the average protein molecular weight was 36,159.25. All identified GGPS genes encoded amino acids ranging from 131 to 421, with an average amino acid length of 329.08. The theoretical isoelectric point of these proteins ranged from 4.22 to 7.84, and the average isoelectric point was 5.94, which was weakly acidic. To understand the expression location of the family, the subcellular localization was predicted (Fig. 1). The results showed that almost all GGPS proteins were expressed in the chloroplast, mitochondria and cytoplasm. It was suggested that GGPS family members play different functions in different cell parts. For example, members of the GGPS family located in chloroplasts might play an important role in chloroplast photosynthesis.
Analysis of the phylogenetic relationship of the GGPS gene family
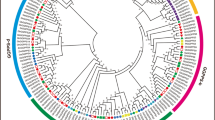
To study the evolutionary relationship among GGPS genes, a phylogenetic tree was constructed using GGPS protein sequences of A. thaliana, G. arboreum, G. raimondii, G. barbadense and G. hirsutum (Fig. 2). The GGPS genes were divided into three subfamilies; the largest branch contained 39 members of the GGPS family in cotton, and the other two branches contained 10 and 26 GGPS family members. It has been speculated that there is a more advanced evolutionary relationship and similar functions for members of the same branch. According to the phylogenetic tree, most orthologous genes between allotetraploids and diploids are clustered closely to each other in the same group, showing expansion of the GGPS gene family in cotton.
Number of phylogenetic relationships of GGPS family genes in Arabidopsis, G. hirsutum, G. barbadense, G. arboreum, and G. raimondii. A Phylogenetic tree of the GGPS gene family in four Gossypium species and Arabidopsis. B Comparisons of GGPS gene numbers in A. thaliana, G. hirsutum, G. barbadense, G. arboreum, and G. raimondii. At: Arabidopsis thaliana; Ga: Gossypium arboreum; Gr: Gossypium raimondii; Gb: Gossypium barbadense; Gh: Gossypium hirsutum
Using the protein sequences, selection pressure was calculated via Calculator 2.0, and the corresponding Ka/Ks values of most of the genes in this family were much less than 1 (Table S2). The rate of synonymous substitution of bases in the development and evolution of most GGPS genes was much higher than that of nonsynonymous substitution, so it was not affected by natural selection. We believe that these genes underwent purifying selection during evolution. There are also some genes with Ka/Ks values greater than 1, such as Gohir.A10G094300 and Gohir.D10G093500, Gohir.D10G093700 and Gohir.D10G093900, Gohir.D10G093700 and Gohir.D10G093800, Gohir.A10G094300 and Gohir.D10G093800, Gohir.D10G093800 and Gohir.D10G093900, Gobar.D10G101700 and Gobar.A10G100400, and Gorai.011G103000 and Gorai.011G102700, indicating that these genes have been positively selected in genetic evolution.
Gene structure and conserved motif analysis of GGPS proteins
To better understand the evolutionary relationship between different members of the GGPS gene family, we constructed phylogenetic trees using the GGPS protein sequence with the NJ method (Fig. 3) and compared and analyzed the intron‒exon structures and conserved motifs of GGPS members of different cotton species. The introns of GGPS genes were different; some GGPS members did not contain introns, while some GGPS genes contained at most 14 introns. The diversity of gene structure indicated that GGPS may have different selection events in the process of gene evolution. Among the four cotton species, the closely related genes in the evolutionary tree tended to have more similar exon and intron arrangements, indicating that the exon‒intron structure was highly related to the phylogenetic relationship between GGPS genes.
Phylogenetic tree, conserved motifs and gene structure analysis of the GGPS family in G. arboreum (A), G. raimondii (B), G. hirsutum (C) and G. barbadense (D). Note: The phylogenetic tree was constructed with MEGA 7 using the neighbor–joining (NJ) method with 1000 bootstrap replicates. The conserved motifs in the GGPS proteins were identified with MEME software. Gray lines denote the nonconserved sequences, and each motif is indicated by a colored box. The lengths of motifs in each protein are presented proportionally. The exon‒intron structures of the GGPS genes are based on the evolutionary relationships. The green rectangle represents exons, and the gray line represents introns
Conserved motifs are often related to the function of proteins. To reveal the characteristic motifs of GGPS, the conserved motifs in GGPS proteins were identified by MEME software. A total of 10 conserved motifs were identified, named Motif1 to Motif10, and the number of conserved motifs in each GGPS varied from 1 to 8 (Fig. 3). Deletions of different motifs were found in all 75 members of the GGPS family, but all GGPS genes had a conservative motif distribution pattern, e.g., motif 2 was found in all proteins, indicating that it was highly conserved in GGPS. In summary, upon analysis of the evolutionary tree, gene structure and conserved motifs, it was found that the GGPS members located in the same branch of the evolutionary tree contain similar gene structures and that the composition and arrangement of their conserved motifs are the same. We speculated that these proteins with similar gene structures and motifs may share similar functions and play similar roles in cotton.
Location and collinearity analysis of GGPS genes on chromosomes
The location distribution map of GGPS on the chromosomes of four cotton species was drawn using TBtools software (Fig. 4). The results showed that among the 27 members of the GGPS family in G. hirsutum, 12 genes were distributed on 6 chromosomes of the At subgenome, which were A01, A05, A07, A10, A11, and A13, and the other 15 genes were distributed on 7 chromosomes of the Dt subgenome, which were D01, D02, D05, D09, D10, D11 and D13. There were 6 pairs of homologous chromosomes on a total of 13 chromosome pairs. In the genome of G. barbadense, we also found that the GGPS gene has a similar distribution on chromosomes. Among the 22 members of the GGPS gene of G. barbadense, 8 were distributed on chromosomes A01, A05, A10, A11, and A13 of the At subgenome, and 14 were distributed on chromosomes D01, D02, D05, D10, D11, and D13 of the Dt subgenome. Thus, the genomes of G. hirsutum and G. barbadense may have come from the same ancestor, and the GGPS gene family is relatively conserved in evolution.
In the diploid cotton G. arboreum, 12 GGPS genes were distributed on chromosomes A01, A02, A05, A07, A08, A11, and A13. In G. raimondii, which is also a diploid species, 14 GGPS members were distributed on chromosomes D02, D05, D07, D09, D11, and D13. Collinearity analysis can well explain the homology between genes, and the collinear homologous sequences may have similar functions, so the collinearity of the GGPS gene family in four different cotton species was analyzed and plotted by MCScanX and Circos software (Fig. 5). We found that the collinearity of GGPS genes in G. raimondii mainly occurred between chromosomes D07 and D11 (Fig. 5B). In the G. arboreum genome, the collinear region of GGPS genes was between chromosomes A10 and A11 (Fig. 5A). In tetraploid cotton species of G. hirsutum and G. barbadense, the collinear relationship between genes mostly occurred between homologous chromosomes (Fig. 5C-D). At the same time, there was a collinear relationship between chromosomes A10 and A11 in the two tetraploid cotton species, which was similar to the collinear region of the GGPS family in the G. arboreum genome.
Sequence alignment and three-dimensional structure prediction of GGPS proteins
The GGPS family is a kind of polypentene synthase in plants. To further determine the sequence characteristics of the cotton GGPS domain, 75 members of the GGPS family were selected for protein sequence alignment and analysis (Fig. 6). The alignment results showed that 75 members of the GGPS family contain two aspartic acid-rich domains: DdxxxxD and DDxxD (x is an arbitrary amino acid), which are typical polypentene synthase domains and beneficial to the binding of IPP and DMAPP and the substrate of GGPS and determine the catalytic activity of GGPS [24].However, a small number of GGPS members had different degrees of deletion of this domain, which might lead to changes in the biological function of these genes. The conformation of proteins is often related to their function. To further understand the function of cotton GGPS proteins, their three-dimensional (3D) structures were predicted through the SWISS-MODEL website (Fig. 7). The results showed that 75 GGPS proteins were mainly composed of α-helices and random crim**, and there was no β-folding. The α-helix is a large number of structural elements in the GGPS polypeptide chain and is scattered in the whole peptide chain. According to protein sequence alignment, it was found that the two functional domains were located in random coils. In addition, there are also some proteins whose 3D structure is too different, such as Gohir.D02G106300. This might be due to the differentiation of these genes in evolution, but the 3D structures of the other members were similar, and similar structures often had similar functions. In addition, GGPS is relatively conserved in the process of evolution.
Analysis of cis-acting elements of GGPS genes in cotton
To understand the potential function of the GGPS gene family, the promoter sequences 1500 bp upstream of GGPS genes were analyzed to detect cis-acting elements (Fig. 8). The results showed that there were many cis-acting elements involved in the physiological process in the upstream promoter region of GGPS genes. There were a large number of cis-acting elements related to light reactions, such as the GA-motif, G-box, TCT-motif, GATA-motif, and GT1-motif. The GGPS gene family may play an important role in the photosynthetic pathway. Of course, there were also cis-acting elements related to abiotic stress responses, such as MYB, ABRE and MBS, in the upstream promoter of the GGPS gene. These results suggested that GGPS genes may also be involved in light response and other physiological processes of biotic and abiotic stresses.
Virus-induced GGPS gene silencing leads to albinism in leaves
Virus-induced gene silencing is an effective means to study gene function. To explore the role of GGPS family members in the growth and development of cotton, a VIGS vector was constructed to silence Gohir. A13G151300 in G. hirsutum "CRI 12" (Fig. 9A). After approximately 2 weeks of Agrobacterium tumefaciens infection, the new true leaves of TRV2:CLA1 showed an albino phenotype. This shows that the VIGS program is correct and effective. Then, we took leaf samples and extracted RNA from TRV2:Gohir. A13G151300 leaves to detect the silencing efficiency. When we compared the silenced gene and TRV2:00 vector as a control, the expression level of this gene was significantly suppressed in TRV2:Gohir.A13G151300 plants, indicating that it was silenced successfully (Fig. 9B). We found that the plant growth of TRV2:Gohir. A13G151300 was significantly slower than that of the WT and TRV2:00, and we also found leaf whitening in TRV2:Gohir.A13G151300. We measured the relative chlorophyll content of the WT, TRV:00 and experimental groups. We measured the relative chlorophyll content of WT, TRV2:00 and TRV2:Gohir.A13G151300. The results showed that the chlorophyll content of TRV2:Gohir.A13G151300 plants decreased significantly (Fig. 9C). These results suggested that the Gohir.A13G151300 gene may be involved in the synthesis of photosynthetic pigments in cotton, and the silencing of the gene leads to damage to the photosynthetic system, which leads to leaf albinism and poor growth.
Virus-induced silencing of the GGPS gene in G. hirsutum. A Phenotypic changes in normal culture of TRV2: Gohir.A13G151300 and control plants after three weeks of injection. B Relative Gohir.A13G151300 transcript levels in leaves of TRV2: Gohir.A13G151300 and control plants (TRV2:00). C Detection of chlorophyll content in leaves of Wildtype, TRV2:00 and TRV2:Gohir.A13G151300 plants. Note: Error bars are the means of three replicates ± SD; T test was used for significance test
Discussion
GGPS is an isoprene pyrophosphate synthase ubiquitous in plants, animals and bacteria. GGPP, synthesized by GGPS, is the precursor of many diterpenes and polyterpenes [1, 3]. GGPP can be used as a substrate to participate in various secondary metabolic pathways, including the synthesis of photosynthetic pigments (chlorophyll and carotenoids) [25]. However, to date, there has been no systematic research or analysis of the GGPS gene family in cotton. Cotton is not only an important fiber crop but also one of the main cash crops in China [26]. With the completion of cotton genome sequencing and the development of plant genetics, we can systematically study the structure, location, function and other information about the cotton GGPS gene family. This paper provides basic biological information for further study of the function of the GGPS gene in cotton.
The formation of the gene family may be due to the whole genome duplication event or polyploidy, which is a large-scale chromosome doubling event that increases the number of all genes in a species at once, resulting in the retention of many chromosome doubling fragments in the genome [27]. Allotetraploid cotton evolved from genomic hybridization and subsequent polyploidy of G. arboreum and G. raimondii [64]. By comparing the sequences of all GGPS proteins, the repeatability and collinearity of GGPS proteins in the cotton genome were determined and analyzed by Multiple Collinearity Scan toolkit (MCSCANX) [65] software.
Cis-acting element analysis
To explore the related functions of gene expression regulation, the promoter sequences 1500 bp upstream of the start codon were obtained from the G. hirsutum genome file, and the cis-acting elements of the genes were analyzed. We identified and analyzed the cis-acting elements of the genes by using the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [66], and the results were mapped using the GSDS online website (http://gsds.gao-lab.org/).
Multi-sequence alignment and three‑dimensional prediction of the protein structure
To analyze the conserved domain of GGPS proteins, ClustalW from MEGA7 [67] was used for multi-sequence alignment of all protein sequences, and then the conserved sequences of the GGPS gene family were calculated and analyzed by GeneDoc software. To further analyze the protein structure of the GGPS gene family, the 3D structure was predicted according to the GGPS protein sequence. The 3D protein models were constructed on the SWISS-MODEL website (https://swissmodel.expasy.org/) [68] using the homologous protein modeling method.
Virus-induced gene silencing (VIGS)
The upland cotton line “ CRI12” was selected for VIGS material. Full and similar seeds were soaked in dilute hydrochloric acid, sterilized, and then planted in flowerpots mixed with nutritious soil and vermiculite at 3:1. The temperature of the greenhouse was 25 °C and the light-to-dark ratio was 16 h: 8 h. The VIGS experiment was carried out when the cotyledons of cotton were completely flattened and the first true leaves of cotton had just appeared. The primers designed by Primer Premier 5 were used for the VIGS reaction and ligated to the pTRV2 vector to obtain the recombinant expression vector. A gene Gohir.A13G151300 was transformed by forward (GCCTCCATGGGGATCCCAAAGTTGTAGCCGATGACC) and reverse (CGAGACGCGTGAGCTCTGCCTGCTTAATCTCACCAC) primer sequences into the pTRV vector using the enzymes Sacl and BamHI to develop pTRV2: Gohir.A13G151300. Then, the plasmid was transformed into Agrobacterium tumefaciens (GV3101). After screening the positive clones, the bacterial solution was injected into the leaves of cotton seedlings with a sterile syringe.
Chlorophyll content determination and RT-qPCR analysis
After the appearance of the albino phenotype in the positive seedling stage, the leaves from the same part of cotton were collected to measure the relative content of chlorophyll and extract total RNA. Chlorophyll content was measured using a SPAD meter at the three true leaf stages. We took the average of three measurements as one replication from a single seedling and with a total of three biological replications. Wild-type and pTRV2:00 were used as negative controls while the Cloroplastos alterados 1 gene (CLA1) was used as a positive control.
A ChamQ SYBR qPCR Master Mix (LowROX Premixed) kit was used for real-time quantitative PCR analysis. Primer Premier 5 was used to design RT-qPCR primers for the GGPS gene family. The reaction volume was 20 µL, and the amplification procedures were as follows: pre-denaturation at 95 °C for 30 s, denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s, and 40 cycles [69]. Each set was replicated three times biologically and technically. Histidine 3 was used as a control, the relative gene expression levels were quantified by 2−ΔΔCt, and the significance was tested by T test [70].
Availability of data and materials
All the data generated in the study are available publicly in the Phytozome database of the Gossypium hirsutum v2.1 genome BioProject, accession numbers of PRJNA515894 and PRJNA713846.
Abbreviations
- GGPS:
-
Geranylgeranyl pyrophosphate synthase
- GGPP:
-
Geranylgeranyl pyrophosphate
- RT-qPCR:
-
Quantitative real-time polymerase chain reaction
- GSDS:
-
Gene Structure Display Server
- MW:
-
Molecular weight
- PI:
-
Theoretical isoelectric point
- HMM:
-
Hidden Markov model
- MCScanX:
-
Multiple Collinearity Scan toolkit
- WGD:
-
Whole genome duplication
- VIGS:
-
Virus-induced gene silencing
- IPP:
-
Isopentenyl pyrophosphate
- DMAPP:
-
Dimethyl allyl pyrophosphate
References
Bouver F, Rahier A, Camara B. Biogenesis, molecular regulation and function of plant isoprenoids. Prog Lipid Res. 2005;44(6):357–429.
Beck G, Coman D, Herren E, Ruiz-Sola MA, Rodríguez-Concepción M, Gruissem W, et al. Characterization of the GGPP synthase gene family in Arabidopsis thaliana. Plant Mol Biol. 2013;82(4–5):393–416.
Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3(7):408–14.
Cazzonelli CI, Pogson BJ. Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010;15(5):266–74.
DellaPenna D, Pogson BJ. Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol. 2006;57:711–38.
Ryan PM, Nigel EG, Amanda GG, Silin Z, Takayuki T, Zhang JF, Alisdair RF, James JG. Manipulation of ZDS in tomato exposes carotenoid- and ABA-specific effects on fruit development and ripening. Plant Biotech J. 2020;18(11):2210–22.
Kuntz M, Römer S, Suire C, Hugueney P, Weil JH, Schantz R, Camara B. Identification of a cDNA for the plastid-located geranylgeranyl pyrophosphate synthase from capsicum annuum: correlative increase in enzyme activity and transcript level during fruit ripening. Plant J. 1992;2(1):25–34.
Xu ZY, He JS, Azhar MT, Zhang Z. UDP-glucose pyrophosphorylase: genome-wide identification, expression and functional analyses in Gossypium hirsutum. Peer J. 2022;10:e13460.
Wang CY, Chen QW, Fan DJ, Li JX, Wang GD, Zhang P. Structural analyses of short chain prenyltransferases identify an evolutionarily conserved GFPPS Clade in Brassicaceae Plants. Mol Plant. 2016;9(2):10.
Okada K, Saito T, Nakagawa T, Kawamukai M, Kamiya Y. Five geranylgeranyl diphosphate synthases expressed in different organs are localized into three subcellular compartments in Arabidopsis. Plant physiol. 2000;122(4):1045–56.
Rai A, Smita SS, Singh AK, Shanker K, Nagegowda DA. Heteromeric and homomeric geranyl diphosphate synthases from Catharanthus roseus and their role in monoterpene indole alkaloid biosynthesis. Mol Plant. 2013;6(5):1531–49.
Li RJ, Zhang H, He SZ, Zhang H, Zhao N, Liu QC. A geranylgeranyl pyrophosphate synthase gene, IbGGPS, increases carotenoid contents in transgenics sweetpotato. J Integr Agric. 2022;21(9):9.
Ament K, Van Schie CC, Bouwmeester HJ, Haring MA, Schuurink RC. Induction of a leaf specific geranylgeranyl pyrophosphate synthase and emission of (E, E) 4,8,12-trimethyltrideca-1,3,7,11-tetraene in tomato are dependent on both jasmonic acid and salicylic acid signaling pathways. Planta. 2006;224(5):1197–2108.
Jenkins GI. Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol. 2009;60(1):407–31.
Wintermans JF, Mots AD. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. BBA. 1965;109(2):448–53.
Ali F, Qanmber G, Wei Z, Yu D, Li YH, Gan L, et al. Genome-wide characterization and expression analysis of geranylgeranyl diphosphate synthase genes in cotton (Gossypium spp.) in plant development and abiotic stresses. BMC Genom. 2020;(21):561.
Ohnuma S, Suzuki M, Nishino T. Archaebacterial ether-linked lipid biosynthetic gene. Expression cloning, sequencing, and characterization of geranylgeranyl-diphosphate synthase. J Biol Chem. 1994;269(20):14792.
Sandmann G, Misawa N, Wiedemann M, Vittorioso P, Carattoli A, Morelli G, et al. Functional identification of al-3 from Neurospora crassa as the gene for geranylgeranyl pyrophosphate synthase by complementation with crt genes, in vitro characterization of the gene product and mutant analysis. J Photochem. 1993;18(2–3):245–51.
Jiang Y, Proteau P, Poulter D, Ferro-Novick S. BTS1 encodes a geranylgeranyl diphosphate synthase in Saccharomyces cerevisiae. J Biol Chem. 1995;270(37):21793–9.
Chong DY, Chen Z, Guan S, Zhang TY, Xu N, Zhao Y, Li CJ. Geranylgeranyl pyrophosphate-mediated protein geranylgeranylation regulates endothelial cell proliferation and apoptosis during vasculogenesis in mouse embryo. J Genet Genomics. 2021;67(03):300.
Mehari TG, Xu Y, Odongo MR, Jawad UM, Nyangasi KJ, Cai X, et al. Genome wide identification and characterization of light-harvesting Chloro a/b binding (LHC) genes reveals their potential role in enhancing drought tolerance in Gossypium hirsutum. J Cotton Res. 2021;4:15.
Tata SK, Jung J, Kim Y, Choi JY, Jung J, Lee I, Shin Ryu SB. Heterologous expression of chloroplast-localized geranylgeranyl pyrophosphate synthase confers fast plant growth, early flowering and increased seed yield. Plant Biotechnol J. 2015;14:1–11.
Zhou F, Wang CY, Gutensohn M, Jiang L, Zhang P, Zhang D, Dudareva N, Lu S. A recruiting protein of geranylgeranyl diphosphate synthase controls metabolic flux toward chlorophyll biosynthesis in rice. PNAS. 2017;114(26):6866–71.
Liang PH. Reaction kinetics, catalytic mechanisms, conformational changes, and inhibitor design for prenyltransferases. Biochemistry. 2009;48(28):6562–70.
Laskaris G, Bounkhay M, Theodoridis G, Heijden R, Verpoorte R, Jaziri M. Induction of geranylgeranyl diphosphate synthase activity and taxane accumulation in Taxus baccata cell cultures after elicitation by methyl jasmonate. Plant Sci. 1999;147(1):1–8.
Bao Y, Hu GJ, Flagel LE, Salmon A, Bezanilla M, Paterson AH, et al. Parallel upregulation of the profiling gene family following independent domestication of diploid and allopolyploid cotton (Gossypium). PNAS. 2011;108(52):21152–7.
Yu J, Wang J, Lin W, Li S, Li H, Zhou J, et al. The genomes of Oryza sativa: a history of duplications. PLoS Biol. 2005;3(2):38.
Li FG, Fan GY, Lu CR, **ao GG, Zou CS, Russell K, et al. Genome sequence of cultivated G. hirsutum (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat Biotechnol. 2015;33(5):524–U242.
Huang G, Wu ZG, Richard GP, Bai MZ, Li Y, James E, et al. Genome sequence of Gossypium herbaceum and genome updates of Gossypium arboreum and Gossypium hirsutum provide insights into cotton A-genome evolution. Nat Genet. 2020;52(5):516–24.
Mehari TG, Xu Y, Umer MJ, Hui F, Cai X, Zhou Z, Hou Y, Wang K, Wang B, Liu F. Genome-wide identification and expression analysis elucidates the potential role of PFK gene family in drought stress tolerance and sugar metabolism in cotton. Front Genet. 2022;13: 922024.
Holub EB. The arms race is ancient history in Arabidopsis, the wildflower. Nat Rev Genet. 2001;2(7):516–27.
Yan Z, Wu NN, Song WL, Yin GJ, Qi YJ, Yan YM, et al. Soybean (Glycine max) expansin gene superfamily origins: segmental and tandem duplication events followed by divergent selection among subfamilies. BMC Plant Biol. 2014;14(1):93.
Malik WA, Afzal M, Chen XG, Cui RF, Lu XK, Wang S, Wang J, Mahmood I, Ye YY. Systematic analysis and comparison of ABC proteins superfamily confer structural, functional and evolutionary insights into four cotton species. Ind Crops Prod. 2022;117: 114433.
Hurst LD. The Ka/Ks ratio: diagnosing the form of sequence evolution. Trends Genet. 2002;18(9):486–7.
Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4(1):10.
Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, et al. The genome of the mesopolyploid crop species Brassica rapa. Nat Genet. 2011;43(10):1035–9.
Qanmber G, Lu LL, Liu Z, Yu DQ, Zhou KH, Huo P, et al. Genome-wide identification of GhAAI genes reveals that GhAAI66 triggers a phase transition to induce early flowering. J Exp Bot. 2019;70(18):4721–36.
**ao GH, He P, Zhao P, Liu H, Zhang L, Pang CY, Yu JN. Genome-wide identification of the GhARF gene family reveals that GhARF2 and GhARF18 are involved in cotton fibre cell initiation. J Exp Bot. 2018;69(18):4323–736.
Rearick D, Prakash A, McSweeny A, Shepard SS, Fedorova L, Fedorov A. Critical association of ncRNA with introns. Nucleic Acids Res. 2011;39(6):2357–66.
Roy WS, Gilbert W. The evolution of spliceosomal introns: patterns, puzzles and progress. Nat Rev Genet. 2006;7(3):211–21.
Roy SW, Penny D. A very high fraction of unique intron positions in the intron-rich diatom Thalassiosira pseudonana indicates widespread intron gain. Mol Biol Evol. 2007;24(7):1447–57.
Nagel R, Bernholz C, Vranová E, Košuth J, Bergau N, Ludwig S, et al. Arabidopsis thaliana isoprenyl diphosphate synthases produce the C25 intermediate geranylfarnesyl diphosphate. Plant J. 2015;84(5):847–59.
Wang GD, Dixon RA. Heterodimeric geranyl (geranyl) diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. PNAS. 2009;106(24):9914–9.
Hsieh FL, Chang TH, Ko TP, Wang A. Structure and mechanism of an Arabidopsis medium/long-chain-length prenyl pyrophosphate synthase. Plant Physiol. 2011;155(3):1079–90.
Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8(3):217–30.
Xu ZF, Chye ML, Li HY, Xu FX, Yao KM. G-box binding coincides with increased Solanum melongena cysteine proteinase expression in senescent fruits and circadian-regulated leaves. Plant Mol Biol. 2003;51(1):9–19.
Dehesh K, Bruce WB, Quail PH. A trans-acting factor that binds to a GT-motif in a phytochrome gene promoter. Science. 1990;250(4986):1397–9.
Ruiz-Sola MÁ, Coman D, Beck G, Barja MV, Colinas M, Graf A, et al. Arabidopsis geranylgeranyl diphosphate synthase 11 is a hub isozyme required for the production of most photosynthesis-related isoprenoids. New Phytol. 2016;209(1):252–64.
Goodstein DM, Shu SQ, Howson R, Neupane R, Hayes RD, Fazo J, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;D1:D1178–86.
Chen ZJ, Sreedasyam A, Ando A, Song QX, De SL, Hulse-Kemp AM, et al. Genomic diversification’s of five Gossypium allopolyploid species and their impact on cotton improvement. Nat Genet. 2020;52(5):525–33.
Paterson AH, Wendel JF, Gundlach H, Guo H, Jenkins J, ** DC, et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature. 2012;492(7429):423–7.
Zhu T, Liang CZ, Meng ZG, Sun GQ, Meng ZG, Guo SD, Zhang R. CottonFGD: an integrated functional genomics database for cotton. BMC Plant Biol. 2017;17(1):101.
Cunningham F, Allen JE, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, et al. Ensembl 2022. Nucleic Acids Res. 2021;50(D1):988–95.
Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:290–301.
Letunic I, Copley RR, Pils B, Pinkert S, Schultz J, Bork P. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 2006;34:257–60.
Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43:222–6.
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31(13):3784–8.
Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–7.
Mello B. Estimating TimeTrees with MEGA and the TimeTree resource. Mol Biol Evol. 2018;35(9):2334–42.
Subramanian B, Gao SH, Lercher MJ, Hu SN, Chen WH. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47(W1):270–5.
Zhang Z, Li J, Zhao XQ, Wang J, Wong GK, Yu J. KaKs_Calculator: calculating ka and ks through model selection and model averaging. Genom Proteom Bioinform. 2006;04:259–63.
Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:202–8.
Hu B, ** JP, Guo AY, Zhang H, Luo JC, Gao G. GSDS 20: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–7.
Voorrips RE. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93(1):77–8.
Wang YP, Tang HB, Debarry JD, Tan X, Li JP, Wang XY, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van PY, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7 for bigger datasets. Mol Biology Evol. 2016;33(7):1870–4.
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46(W1):296–303.
Sui N, Yang Z, Liu M, Wang B. Identification and transcriptomic profiling of genes involved in increasing sugar content during salt stress in sweet sorghum leaves. BMC genom. 2015;16:534.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8.
Acknowledgements
The authors acknowledge School of Life Sciences, Nantong university for providing the laboratory and input facility for this experiment.
Funding
We appreciate financial help from the National Key R&D Program of China (2021YFE0101200), the Pakistan Science Foundation, PSF/CRP/18thProtocol (07); the National Natural Science Foundation of China (52161145104); Key Research and Development Project of Jiangsu Province, China (Modern Agriculture, BE2022364); State Key Laboratory of Cotton Biology Open Fund (CB2022A02); Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_3330); Project No. PSDP-829; and the Practice Innovation Training Program Projects for College Students (202210304024Z).
Author information
Authors and Affiliations
Contributions
WF, TGM, and HF performed most of the experiments and data analysis. MJ, ZQ, MJ, and DW helped in sample preparation and data analysis. AD, MKRK, and YC helped to design the experiments and revise the manuscript. BW and JW designed the experiments and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the seeds used for planting materials during the experiment were provided by our school and all the procedures were followed in accordance with international guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1.
Data for analysis of the physicochemical properties of GGPS family genes of the four cotton species.
Additional file 2: Supplementary Table S2.
Ka/Ks analysis of the homologous GGPS gene family.
Additional file 3: Supplementary Table S3.
List of genes and their accession IDs for G. hirsutum, G. arboreum, G. raimondii, G. barbadense and A. thaliana.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Feng, W., Mehari, T.G., Fang, H. et al. Genome-wide identification of the geranylgeranyl pyrophosphate synthase (GGPS) gene family involved in chlorophyll synthesis in cotton. BMC Genomics 24, 176 (2023). https://doi.org/10.1186/s12864-023-09249-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-023-09249-w