Abstract
Background
Local Chinese local pig breeds have thinner muscle fiber and higher intramuscular-fat (IMF) content. But its regulation mechanism has not been discussed in-depth. Studies indicated that long non coding RNAs (lncRNAs) play important role in muscle and fat development.
Results
The lncRNAs expressional differences in the longissimus dorsi (LD) muscle were identified between Huainan pigs (local Chinese pigs, fat-type, HN) and Large White pigs (lean-type, LW) at 38, 58, and 78 days post conception (dpc). In total, 2131 novel lncRNAs were identified in 18 samples, and 291, 305, and 683 differentially expressed lncRNAs (DELs) were found between these two breeds at three stages, respectively. The mRNAs that co-expressed with these DELs were used for GO and KEGG analysis, and the results showed that muscle development and energy metabolism were more active at 58 dpc in HN, but at 78 dpc in LW pigs. Muscle cell differentiation and myofibril assembly might associated with earlier myogenesis and primary-muscle-fiber assembly in HN, and cell proliferation, insulin, and the MAPK pathway might be contribute to longer proliferation and elevated energy metabolism in LW pigs at 78 dpc. The PI3K/Akt and cAMP pathways were associated with higher IMF deposition in HN. Intramuscular fat deposition-associated long noncoding RNA 1 (IMFlnc1) was selected for functional verification, and results indicated that it regulated the expressional level of caveolin-1 (CAV-1) by acting as competing endogenous RNA (ceRNA) to sponge miR-199a-5p.
Conclusions
Our data contributed to understanding the role of lncRNAs in porcine-muscle development and IMF deposition, and provided valuable information for improving pig-meat quality.
Similar content being viewed by others
Background
In daily human diet, the main source of animal protein is pork, especially in China, where pork is consumed the most by residents. Recently, more and more attention has been paid to meat quality. The diameter of muscle fibers, the content of intramuscular fat, and muscle fiber types are all involved in regulating meat-quality traits [1, 2]. Meat-quality traits are also regulated by nutrition, feeding conditions, stress, and, most importantly, by genetic influence [3]. Lean-type pigs like Large White (LW), Landrace (LR), and Duroc generally have advantages such as high meat production, a fast growth rate, and a low feed-to-meat ratio. Most local Chinese pig breeds are fat-type, and they generally have bright red meat, thin muscle fibers, high intramuscular fat content, and more glycolytic muscle fibers. Two pig types with differences in meat-quality traits were a potentially good model to study the genetic-regulation mechanism of potential muscle-phenotype differences [4].
Some studies focused on the differences between lean and fat-type pigs recently. The transcriptome of the longissimus dorsi (LD) muscle between German Landrace (more obese) and Pietrain (more leaner) fetuses at embryonic (35, 63, and 91 days post conception (dpc)) and adult period (180 days postnatal) was compared, and results indicated that the differential expressed genes were related with muscle development stages and meat quality [5]. Xu et al. compared the transcriptome of LD muscle between Northeast Min (fat-type) and Changbaishan (lean-type) wild boar at the age of 42 days; the difference between these two breeds was mainly attributed to development of myofiber, and metabolism of lipid [6]. The LD muscle miRNAomes between Meishan (MS) and LW pigs at 35 dpc were compared, and results revealed that, in 87 differentially expressed miRNAs mainly enriched in mammalian target of rapamycin (mTOR), muscle contraction, wingless/integrated (WNT), and mitogen-activated protein kinase (MAPK) signaling pathway, and the highly abundant miRNAs in these two pigs were different [7]. At postnatal day 240, the miRNAs with different expressional level in the LD muscle of LR and Tongcheng (TC) pigs were mainly enriched in the muscle-organ development and oxidative stress [8]. Sun et al., compared the transcriptome of LD muscle between LR and Lantang (LT) pigs at the age of 7 days. They found 547 DEGs, 5566 differentially expressed lncRNAs (DELs), and 4360 differentially expressed circRNAs (DECs), respectively [9]. DNA methylation in TC, LR and Wuzhishan (WZS) pigs’ LD muscle at the age of 240 days were compared, muscle development and lipid metabolism were enriched for the differently methylated genes [10]. Meat quality differences between fat- and lean-type pigs were mainly due to muscle development and lipid metabolism.
In previous studies, LD muscle samples were collected from fetal, new-born, or adult pigs. Zhao et al., identified the characterization of large intergenic noncoding RNAs (lincRNAs) in the LD muscle of TC pigs at 50, 55, 60, 65, and 75 dpc [11], but the expressional differences of lncRNAs during the embryonic stage between fat- and lean-type have not been systematic studied. During the embryonic stage, there are two major fiber generation periods. The first wave is myoblasts differentiating into primary myofibers (35—60 dpc), and the second is the secondary myofibers forming a base on primary myofibers (54—90 dpc) [12]. Before birth, the total number of fibers (TNF) is fixed, for postnatal, the development of muscle is mainly growth and maturation, so the embryonic stage is vital in skeletal muscle development [13]. Researches also showed that adipogenic differentiation and the second wave of fiber generation are simultaneous in cattle. In the later stages of embryonic development, the differences in intramuscular fat between TC and Yorkshire (YK) pigs were significantly [12]. In production, increasing the nutritional level of females in later pregnancy could promote muscle fat content of the young animals. Therefore, the embryonic stage is a key stage for studying meat quality traits.
Recently researches indicated that long non coding RNA (lncRNA) also participated in the regulation of muscle development and lipid metabolism by competing endogenous RNA (ceRNA) mechanism. lncRNA maternally expressed gene 3 (Meg3) induced 3 T3-L1 preadipocytes adipogenesis via miR-217-Dickkopf-3 pathway [14]. In human adipose tissue-derived mesenchymal stem cells (ADSCs), Terminal differentiation-induced ncRNA (TINCR) modulated the adipogenic differentiation by forming a feedback loop with miR-31 and C/EBP-α [15]. A novel lncRNA (muscle anabolic regulator 1, MAR1) promoted muscle differentiation and regeneration by sponging miR-487b and regulating Wnt5a [16]. lncRNA TUG1 promoted VSMCs proliferation and migration via miR-145-5p-FGF10 pathway [17].
As an excellent local breed in China, Huainan (HN) pigs are mainly distributed in the upper reaches of the Huai River, showing heat- and roughage-resistance, and high intramuscular fat deposition. Huainan breed was name “fine livestock and poultry breeds in Henan province” in 1986 [18]. Previously, we studied the characterization of lncRNA in adult porcine subcutaneous fat and LD muscle [19,20,21]. So far, the effect of lncRNAs on the muscle development in the embryo stage of Huainan pigs hasn’t been researched. In this study, considering the temporality of myogenic differentiation, lncRNA expression profiles in LD muscle between HN pigs and LW pigs at 38, 58, and 78 dpc was compared. Considering the expressional level (FPKM > 10), transcript length (< 2000 bp), and miR-199a-5p binding site, lnc_000167 was selected from the 37 shared DELs in different stages, and it was named intramuscular fat deposition-associated long noncoding RNA 1 (IMFlnc1). Results were helpful to analyze the lncRNAs’ regulation mechanism on differences of meat quality between fat- and lean-type pigs during the embryonic period, and provide basic materials for improving meat quality traits for breeding.
Results
lncRNA identification in porcine LD muscle
To verify the effect of lncRNAs in LD muscle development during porcine embryonic period, we examined the DELs of LD muscles between HN and LW pigs at the three embryonic-development stages. In total, there were 1.94 billion clean reads in these 18 samples, and 79.63% mapped to the porcine reference genome (Sscrofa10.2), 12.87% were multiply mapped, 66.76% were uniquely mapped, 33.34% mapped to “+”, 33.47% mapped to “–”, 19.24% were splice reads, and 47.52% was nonsplice reads (Additional file 1: Table S1). Expression correlation between the three samples in the same treatment was from 0.953 to 0.969 (Additional file 5: Figure S1), indicating that sample selection was reasonable, and experiment results were reliable.
lncRNA properties in porcine LD muscle
In total, 2131 novel lncRNAs and 2057 annotated lncRNAs were detected from 18 LD muscle samples (Fig. 1a), and only lincRNA (89.9%)and intronic lncRNA were found in the novel lncRNAs (Fig. 1b). The annotated lncRNAs’ average length was shorter than the novel lncRNAs’ average length, but there was no significant difference in open reading frame (ORF) length and exon number (Fig. 1c). For the novel lncRNAs, antisense lncRNAs’ average length was longer than lincRNAs’ average length (Fig. 1d). Information on the novel lncRNAs is shown in Additional file 2: Table S2.
Genomic features of the identified lncRNAs in longissimus dorsi muscle tissue of HN and LW pigs at 35, 55 and 75 days post-conception. a Screening of candidate lncRNAs by analyzing their coding potential via CPC, CNCI, phyloCSF and PFAM. b The classification of the 2131 novel identified lncRNAs. c Length, exon number and ORF length distribution of annotated lncRNAs, novel lncRNAs and mRNAs. d Length, exon number and ORF length distribution of novel discovered antisense lncRNAs and lincRNAs
Expression difference of lncRNAs between HN and LW pigs
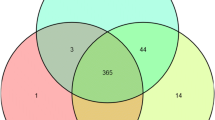
The expressional level of lncRNAs were lower than that of mRNAs (Fig. 2a), and lncRNAs FPKM distribution of LW pigs at 78 dpc was higher than that of the others (Fig. 2b). Systematic cluster analysis was carried out to analyze the 18 LD muscle libraries’ relationship, and results indicated that three replicates of the same sample were very conservative (Fig. 2c). The lncRNAs expression differences between the different stages were larger than those of the two breeds. There were 1155 and 751 DELs during muscle development in HN and LW pigs, respectively. In HN pigs, 579 and 809 DELs were identified at 58 vs 38 dpc and 78 vs 58 dpc, and 213 DELs were shared in these two comparisons. In LW pigs, there were 579 and 809 DELs at 58 vs 38 dpc and 78 vs 58 dpc, respectively, and 167 DELs were shared. At 78 vs 58 dpc, there were more DELs in HN than in LW pigs. There were 291, 305, and 683 DELs between these two breeds at 38, 58, and 78 dpc, and 37 DELs were shared by these three stages (Fig. 2d and Additional file 6: Figure S2 and Additional file 3: Table S3).
The differentially expressed lncRNAs in longissimus dorsi muscle tissue between Huainan (HN) and Large white (LW) at 38, 58 and 78 post-conception. a The FPKM distribution of lncRNAs and mRNAs. b FPKM distribution of lncRNAs in HN and LW. c Hierarchical clustering of differentially expressed lncRNAs. d The numbers of DELs between different stages in HN or LW were depicted on vertical lines. The numbers of DELs between HN and LW at each stage were depicted on horizontal lines. ↑: up-regulated, ↓: down-regulated
During embryonic muscle development, the GO enrichment of DEL-co-expressed genes in HN showed that muscle tissue development and the response to oxygen-containing compound were upregulated in 58 dpc. In LW pigs, only the response to the oxygen-containing compound was upregulated at 58 dpc, and muscle cell differentiation, myofibril assembly were only upregulated at 78 dpc; other muscle development-related pathways were continuously upregulated in both breeds. At 58 vs 38 dpc, muscle cell differentiation and myofibril assembly were only upregulated in HN at 58 dpc. Skeletal muscle tissue development was only upregulated in LW at 78 dpc (Table 1 and Additional file 3: Table S3).
KEGG enrichment of DEL-co-expressed genes shown that the cyclic adenosine monophosphate (cAMP), glyoxylate and dicarboxylated metabolism, metabolic pathways were only upregulated in HN pigs at 58 dpc. Fatty acid degradation and fructose and mannose metabolism was only upregulated in LW pigs at 78 dpc. The adipocytokine pathway was upregulated in HN but downregulated in LW pigs at 58 dpc. The MAPK pathway was upregulated only at 58 dpc in both breeds, and fructose and mannose metabolism was upregulated only at 78 dpc in both breeds. DEL-co-expressed genes related to fatty acid degradation, cAMP, glyoxylate and dicarboxylated metabolism, fatty acid metabolism, metabolic pathways continuously changed only in HN or LW pigs. Genes associated with metabolism of alanine, aspartate and glutamate, and Rap1 with higher expressional level in HN pigs at 78 dpc, while genes in protein digestion and absorption with a higher level in LW pigs at 78 dpc (Table 2 and Additional file 7: Figure S3).
The results of GO enrichment of DELs between HN and LW pigs at the same stage showed that no pathway was enriched between these two breeds at 38 dpc. At 58 dpc, genes in muscle cell differentiation and eight other pathways had a lower expression level in LW pigs. At 78 dpc, the genes in seven pathways were upregulated in LW pigs. Genes associated with muscle cell differentiation, skeletal muscle tissue/organ development, muscle organ development pathways showed, higher expressional level at 58 dpc, but a lower expression level in HN pigs than that of in LW pigs (Table 1 and Additional file 3: Table S3).
KEGG analysis indicated that DEL-co-expressed genes involved in insulin, calcium, and the cAMP signaling pathway were continuously highly expressed in HN pigs. Genes in steroid biosynthesis showed a higher level in HN pigs at 58 and 78 dpc. Compared with LW pigs, genes in hypoxia-inducible factor 1 (HIF-1) and adipocytokine signaling pathway were upregulated in LW pigs at 38 and 78 dpc, but downregulated at 58 dpc. Genes involved in insulin secretion and Rap1 were downregulated at 58 dpc and upregulated at 78 dpc in HN pigs. The expression trends of biosynthesis of amino acids, fructose and mannose metabolism, and fatty acid metabolism were completely opposite (Table 2 and Additional file 7: Figure S3).
Potential function of DELs
The expressional level of 37 shared DELs between these two breeds at three stages are shown in Fig. 3a and b. The ceRNA regulatory network of these shared DELs was constructed and visualized using Cytoscape software, including 30 lncRNAs, 27 miRNAs, 27 mRNAs, and 24 pathways (Fig. 3c). LncRNAs had up to 7 interacting miRNAs, such as ALDBSSCT0000006192, and miR-199a-5p had the most target lncRNAs, seventeen target lncRNAs for each miRNA. Considering the abundance and transcript length, IMFlnc1 was selected for subsequent verification among miR-199a-5p target lncRNAs. IMFlnc1 is located on porcine chromosome 7, and includes two exons. The IMFlnc1 expressional level in intramuscular adipose tissue was higher than that in LD muscle tissue (Fig. 4a), so it was speculated that IMFlnc1 might play an important role in intramuscular adipose tissue, so we firstly verified its regulation role in adipogenesis of porcine intramuscular adipocytes.
The function of 37 shared DELs between HN and LW at each stage. a Venn diagram showing the number of overlap** DELs between HN and LW at three stages. b The heatmap of the 37 shared DELs’ expressional level in three stages between HN and LW pigs. Red arrow showed the selected LNC_000167 (IMFlnc1). c The ceRNA network of 37 shared DELs. Circular nodes represent lncRNAs; triangular nodes represent miRNAs; diamond nodes represent mRNAs; and square nodes represent pathways
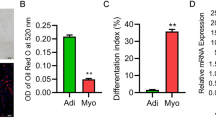
IMFlnc1 was associated with adipogenesis. a The expression profile of IMFlnc1 in different tissues by qRT-PCR. b The expression level of IMFlnc1 and PPARgama during porcine intramuscular preadipocyte differentiation by qRT-PCR. c The coding probability of IMFlnc1, ADNCR and CAV-1 were analyzed by Coding Potential Calculator 2 (CPC2) program. d IMFlnc1 siRNA could downregulate expression of IMFlnc1, CAV-1 and PPARgama by qRT-PCR. e The effect of IMFlnc1 siRNA on CAV-1 protein expression by Western blot. f Inhibition of IMFlnc1 inhibited adipogenesis by Oil Red O staining
IMFlnc1 promotes adipogenesis of intramuscular adipocytes
RT-qPCR showed that IMFlnc1 showed the highest expressional level in the gut and lungs, followed by in intermuscular fat. Its expression level in subcutaneous fat was lower than it was in intermuscular fat, but higher than it was in LD muscle (Fig. 4a). Moreover, time-course analysis showed that IMFlnc1, CAV-1 and PPARgama were upregulated during the differentiation of porcine intramuscular preadipocyte, which was similar with PPARgama expressional trends (Fig. 4b).
Analyzed by CPC software, IMFlnc1 showed very low coding potential, similar to a well-known lncRNA—ADNCR [22], (Fig. 4c). To explore the function of IMFlnc1 in adipogenesis, we performed knockdown IMFlnc1 in intramuscular adipocytes by lncRNA smart silencer, its RNA level was significantly reduced, and CAV-1 and PPARgama (adipogenic markers) significantly downregulated (Fig. 4d). The downregulation of CAV-1 by IMFlnc1 siRNA was confirmed by Western blot (Fig. 4e). Oil Red O staining indicated that adipogenesis was inhibited by knockdown of IMFlnc1 (Fig. 4f).
IMFlnc1 and CAV-1 were miR-199a-5p’s target genes
IMFlnc1 is mainly localized in preadipocyte cytoplasm (Fig. 5a), so it might participate in the regulation of adipogenesis through ceRNA mechanism. The IMFlnc1-miR-199a-5p-CAV-1 pathway was selected from the ceRNA network to verify its function in adipogenesis. Bioinformatics analysis of the RNAhybrid showed that there exists a binding site of miR-199a-5p in IMFlnc1 and CAV-1 (Fig. 5b), and the binding site in CAV-1 is conservative in different animals (Fig. 5c). The results of RT-qPCR showed that the expressional trends of IMFlnc1 and CAV-1 has a positive correlation in porcine LD muscle (Fig. 5d, R2 = 0.590).
IMFlnc1 regulates CAV-1 expression by sponging miR-199a-5p. a Detection of IMFlnc1 in porcine intramuscular adipocyte by RNA fluorescence in situ hybridization (RNA-FISH). Red represents FISH probes of IMFlnc1. Nuclei are counterstained with DAPI (blue). Scale bar, 50 μm. b The schematic diagram shows the sequences of IMFlnc1 and CAV-1 with miR-199a-5p, inculding wild-type (Wt) and mutant type (Mut). c The binding site of miR-199a-5p at CAV-1 3’UTR (green) are evolutionarily conserved across species. d The correlation analysis of IMFlnc1 expression level and CAV-1 mRNA level in 18 porcine LD muscle tissues. e IMFlnc1 acted as the target of miR-199a-5p. f CAV-1 acted as the target of miR-199a-5p. g IMFlnc1 acted as the sponge of miR-199a-5p. IMFlnc1 increases CAV-1 expression (h) and adipogenesis (i) in a miR-199a-5p dependent manner
Luciferase assay indicated that miR-199a-5p significantly reduced the fluorescence activity of the sensor and psiCHECK2-IMFlnc1 (p < 0.01, Fig. 5e). However, miR-199a-5p couldn’t reduce the fluorescence activity of psiCHECK2-IMFlnc1-Mut, which indicated that miR-199a-5p target IMFlnc1. Similarly, miR-199a-5p also target CAV-1 (Fig. 5f).
IMFlnc1 participates in adipogenesis by increasing CAV-1
To verify whether IMFlnc1 might sponge miR-199a-5p, the miR-199a-5p sensor was transfected with pcDNA3.1+, pcDNA-miR-199a-5p or pcDNA-IMFlnc1. The pcDNA-miR-199a-5p significantly reduced the fluorescence activity of the miR-199a-5p sensor (p < 0.01), but the fluorescence activity could be recovered by IMFlnc1 in a dose-dependent manner (p < 0.01, Fig. 5g). However, pcDNA-IMFlnc1-Mut (the binding site of miR-199a-5p was mutated) couldn’t recover the fluorescence activity. It was indicated that IMFlnc1 could sponge miR-199a-5p.
From Fig. 4d and e, mRNA and protein of CAV-1 was downregulated during the reduction of IMFlnc1. To further determine whether IMFlnc1 regulated CAV-1 through miR-199a-5p, psiCH2-CAV-1 was co-transfected with pcDNA3.1, pcDNA-IMFlnc1 or pcDNA-IMFlnc1Mut, respectively. The Rluc activity of CAV-1 was improved by the overexpression of IMFlnc1, but the overexpression of IMFlnc1 with the mutated miR-199a-5p binding sites no longer elicited similar effect (Fig. 5h). And Oil Red O staining confirming that, compared with inhibition of IMFlnc1, the number of mature adipocyte was increased after inhibition of miR-199a-5p and IMFlnc1 (Fig. 5i). In summary, these results indicated that IMFlnc1 might promote adipogenesis by sponging miR-199a-5p.
Discussion
Previous studies showed that muscle development during the embryonic stages is different between lean- and fat-type pigs. The LD muscle morphological differences between LT and LR pigs were compared by histological section, primary fibers could be found at 35 dpc in LT pigs, but in LR pigs primary fibers could be found at 49 dpc. The secondary fibers formed at 63 dpc in both breeds, LR pigs had more fibers and larger muscle fiber diameter at 91 dpc [Luciferase assays HEK293T cells were cultured in 48-well plates, and plasmids were transfected when the cell reached 70% or 80% confluence. Luciferase activities were measured 48 hours after transfection on a Fluoroskan Ascent FL instrument (Thermo Fisher Scientific, USA) by using the Dual-Luciferase Reporter Assay System (Promega). The relative Rluc activity for each sample was calculated by normalized renilla luciferase activity to firefly luciferase activity. Results were representing as mean ± SE, and it was considered statistically significant when the p value < 0.05.Statistical analysis
Availability of data and materials
The datasets used in current study are available in the manuscript and its additional files, and raw sequence data had been submitted to NCBI Sequence Read Archive (SRP243554).
Abbreviations
- ADD1:
-
Adipocyte determination and differentiation dependent factor 1
- cAMP:
-
Cyclic adenosine monophosphate
- CAV-1:
-
Caveolin-1
- C/EBPα:
-
Ccaat enhancer binding protein-α
- ceRNA:
-
Competing endogenous RNA
- CNCI:
-
Coding-Non-Coding-Index
- CPC:
-
Coding Potential Calculator
- DEGs:
-
Differentially expressed genes
- DELs:
-
Differentially expressed lncRNAs
- Dnm3os:
-
DNM3 opposite strand/antisense RNA
- dpc:
-
Days post conception
- EBF1:
-
Early B cell factor 1
- ERK:
-
Extracellular signal-regulated kinase
- FABP4:
-
Fatty acid binding protein 4
- FPKM:
-
Fragments per kilobase per million
- GLUT4:
-
Glucose transporter 4
- GPR G:
-
Protein-coupled receptor
- HIF-1α:
-
Hypoxia inducible factor-1α
- HK2:
-
Hexokinase 2
- HN:
-
Huainan pigs
- IGF-I:
-
Insulin-like growth factor I
- IMF:
-
Intramuscular-fat
- IMFlnc1:
-
Intramuscular fat deposition-associated long noncoding RNA 1
- JunB:
-
JunB proto-oncogene
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LD:
-
Longissimus dorsi
- lincRNAs:
-
Large intergenic noncoding RNAs
- lncRNAs:
-
Long non coding RNAs
- LR:
-
Landrace
- LT:
-
Lantang
- LW:
-
Large White
- MAPK:
-
Mitogen-activated protein kinase
- Mef2dL:
-
Myocyte-specific enhancer factor 2D
- MS:
-
Meishan
- mTOR:
-
Mammalian target of rapamycin
- ORF:
-
Open reading frame
- Pi:
-
Pietrain
- PI3K/Akt:
-
Phosphatidylinositol-3-kinases/protein-serine-threonine kinase
- PRDM16:
-
PR domain-containing 16
- RIN:
-
RNA integrity number
- RNA FISH:
-
RNA fluorescence in situ hybridization
- SCD:
-
Stearoyl-CoA desaturase
- TC:
-
Tongcheng
- TGFBI:
-
Transforming growth factor beta induced
- TNF:
-
Total number of fibers
- WNT:
-
Wingless/integrated
- WZS:
-
Wuzhishan
- YK:
-
Yorkshire
References
Joo ST, Kim GD, Hwang YH, Ryu YC. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95(4):828–36.
Gao SZ, Zhao SM. Physiology, affecting factors and strategies for control of pig meat intramuscular. Recent Pat Food Nutr Agric. 2009;1(1):59–74.
Piush K, Christian M, Clint S, Kent G, Francesco T. Genetic parameters of meat quality, carcass composition, and growth traits in commercial swine. J Anim Sci. 2019;97(9):3669–83.
Sun YM, Chen XC, Qin J, Liu SG, Zhao R, Yu TY, et al. Comparative analysis of long noncoding RNAs expressed during intramuscular adipocytes adipogenesis in fat-type and lean-type pigs. J Agric Food Chem. 2018;66(45):12122–30.
Siengdee P, Trakooljul N, Murani E, Schwerin M, Wimmers K, Ponsuksili S. Transcriptional profiling and mirna-dependent regulatory network analysis of longissimus dorsi muscle during prenatal and adult stages in two distinct pig breeds. Anim Genet. 2013;44:398–407.
Xu XX, Mishra B, Qin N, Sun X, Zhang SM, Yang JZ, et al. Differential transcriptome analysis of early postnatal develo** longissimus dorsi muscle from two pig breeds characterized in divergent myofiber traits and fatness. Anim Biotechnol. 2019;30:63–74.
He DT, Zou TD, Gai XR, Ma JD, Li MZ, Huang ZQ, et al. Microrna expression profiles differ between primary myofiber of lean and obese pig breeds. PLoS One. 2017;12:e0181897.
Hou XH, Yang YY, Zhu SY, Hua CJ, Zhou R, Mu YL, et al. Comparison of skeletal muscle mirna and mrna profiles among three pig breeds. Mol Gen Genomics. 2016;291:559–73.
Sun JJ, **e M, Huang Z, Li H, Chen T, Sun R, et al. Integrated analysis of non-coding rna and mrna expression profiles of 2 pig breeds differing in muscle traits. J Anim Sci. 2017;95:1092–103.
Yang YL, Liang GM, Niu GL, Zhang YY, Zhou R, Wang YF, et al. Comparative analysis of DNA methylome and transcriptome of skeletal muscle in lean-, obese-, and mini-type pigs. Sci Rep. 2017;7:39883.
Zhao WM, Mu YL, Ma L, Wang C, Tang ZL, Yang SL, et al. Systematic identification and characterization of long intergenic non-coding rnas in fetal porcine skeletal muscle development. Sci Rep. 2015;5:8957.
Zhao YQ, Li J, Liu HJ, ** Y, Xue M, Liu WH, et al. Dynamic transcriptome profiles of skeletal muscle tissue across 11 developmental stages for both Tongcheng and Yorkshire pigs. BMC Genomics. 2015;16:377.
Wigmore PM, Stickland NC. Muscle development in large and small pig fetuses. J Anat. 1983;137:235–45.
Huang XW, Fu CH, Liu WH, Liang YS, Li PL, Liu ZQ, et al. Chemerin-induced angiogenesis and adipogenesis in 3 T3-L1 preadipocytes is mediated by lncRNA Meg3 through regulating Dickkopf-3 by sponging miR-217. Toxicol Appl Pharmacol. 2019;385:114815.
Liu YT, Wang YQ, He XF, Zhang S, Wang K, Wu HJ, et al. LncRNA TINCR/miR-31-5p/C/EBP-α feedback loop modulates the adipogenic differentiation process in human adipose tissue-derived mesenchymal stem cells. Stem Cell Res. 2018;32:35–42.
Zhang ZK, Li L, Guan DG, Liang C, Zhuo ZJ, Liu J, et al. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J Cachexia Sarcopenia Muscle. 2018;9(3):613–26.
Shi L, Tian CJ, Sun LZ, Cao FF, Meng ZY. The lncRNA TUG1/miR-145-5p/FGF10 regulates proliferation and migration in VSMCs of hypertension. Biochem Biophys Res Commun. 2018;501(3):688–95.
Tao H, Mei SQ, Sun XJ, Peng XW, Zhang XY, Ma CP, et al. Associations of tcf12, ctnnal1 and wnt10b gene polymorphisms with litter size in pigs. Anim Reprod Sci. 2013;140:189–94.
Wang J, Hua LS, Chen JF, Zhang JQ, Bai XX, Gao BS, et al. Identification and characterization of long non-coding rnas in subcutaneous adipose tissue from castrated and intact full-sib pair Huainan male pigs. BMC Genomics. 2017;18:542.
**ng BS, Bai XX, Guo HX, Chen JF, Hua LS, Zhang JQ, et al. Long non-coding rna analysis of muscular responses to testosterone deficiency in Huainan male pigs. Anim Sci J. 2017;88:1451–6.
Wang J, Ren QL, Hua LS, Chen JF, Zhang JQ, Bai HJ, et al. Comprehensive analysis of differentially expressed mrna, lncrna and circrna and their cerna networks in the longissimus dorsi muscle of two different pig breeds. Int J Mol Sci. 2019;20:1107.
Li MX, Sun XM, Cai HF, Sun YJ, Plath M, Li CJ, et al. Long non-coding rna adncr suppresses adipogenic differentiation by targeting mir-204. Biochim Biophys Acta. 1859;2016:871–82.
Zhao X, Mo DL, Li AN, Gong W, **ao SQ, Zhang Y, et al. Comparative analyses by sequencing of transcriptomes during skeletal muscle development between pig breeds differing in muscle growth rate and fatness. PLoS One. 2011;6:e19774.
Rhoads RP, Baumgard LH, El-Kadi SW, Zhao LD. Physiology and endocrinology symposium: roles for insulin-supported skeletal muscle growth. J Anim Sci. 2016;94:1791–802.
Segalés J, Perdiguero E, Muñoz-Cánoves P. Regulation of muscle stem cell functions: a focus on the p38 mapk signaling pathway. Front Cell Dev Biol. 2016;4:91.
Jiao Y, Liang X, Hou J, Aisa Y, Wu H, Zhang Z, et al. Adenovirus type 36 regulates adipose stem cell differentiation and glucolipid metabolism through the pi3k/akt/foxo1/pparγ signaling pathway. Lipids Health Dis. 2019;18:70.
Dong X, Tang S, Zhang W, Gao W, Chen Y. Gpr39 activates proliferation and differentiation of porcine intramuscular preadipocytes through targeting the pi3k/akt cell signaling pathway. J Recept Signal Transduct Res. 2016;36:130–8.
Liu G, Li M, Xu Y, Wu S, Saeed M, Sun C. Colxv promotes adipocyte differentiation via inhibiting DNA methylation and camp/pka pathway in mice. Oncotarget. 2017;8:60135–48.
Siengdee P, Trakooljul N, Murani E, Brand B, Schwerin M, Wimmers K, et al. Pre- and post-natal muscle microrna expression profiles of two pig breeds differing in muscularity. Gene. 2015;561:190–8.
Cai ZW, Zhang LF, Chen ML, Jiang XL, Xu NY. Castration-induced changes in microrna expression profiles in subcutaneous adipose tissue of male pigs. J Appl Genet. 2014;55:259–66.
Prats-Puig A, Ortega FJ, Mercader JM, Moreno-Navarrete JM, Moreno M, Bonet N, et al. Changes in circulating micrornas are associated with childhood obesity. J Clin Endocrinol Metab. 2013;98:E1655–60.
Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A, et al. Mirna expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One. 2010;5:e9022.
Lee JS, Kim JM, Lim KS, Hong JS, Hong KC, Lee YS. Effects of polymorphisms in the porcine microrna mir206 / mir133b cluster on muscle fiber and meat quality traits. Anim Genet. 2013;44:101–6.
Feng Y, Niu LL, Wei W, Zhang WY, Li XY, Cao JH, et al. A feedback circuit between mir-133 and the erk1/2 pathway involving an exquisite mechanism for regulating myoblast proliferation and differentiation. Cell Death Dis. 2013;4:e934.
Liu YK, Li MZ, Ma JD, Zhang J, Zhou CW, Wang T, et al. Identification of differences in microrna transcriptomes between porcine oxidative and glycolytic skeletal muscles. BMC Mol Biol. 2013;14:7.
Chu YX, Yao Y, Zhu YB, Shi XE, Sun SD, Li X. Mir-370 enhances cell cycle and represses lipid accumulation in porcine adipocytes. Anim Biotechnol. 2019:1–9.
Feng YT, Zhou LT, Peng Y, Yang YC, Fan TY, Jiang X, et al. The role of mir-326 in adipogenic differentiation of human adipose-derived stem cells by targeting c/ebpalpha in vitro. Anat Rec. 2019. https://doi.org/10.1002/ar.24281.
Belarbi Y, Mejhert N, Gao H, Arner P, Ryden M, Kulyte A. Micrornas-361-5p and mir-574-5p associate with human adipose morphology and regulate ebf1 expression in white adipose tissue. Mol Cell Endocrinol. 2018;472:50–6.
Cazanave SC, Mott JL, Elmi NA, Bronk SF, Masuoka HC, Charlton MR, et al. A role for mir-296 in the regulation of lipoapoptosis by targeting puma. J Lipid Res. 2011;52:1517–25.
Hashemi Gheinani A, Burkhard FC, Rehrauer H, Aquino Fournier C, Monastyrskaya K. Microrna mir-199a-5p regulates smooth muscle cell proliferation and morphology by targeting wnt2 signaling pathway. J Biol Chem. 2015;290:7067–86.
Yan MJ, Yang SB, Meng FB, Zhao ZH, Tian ZS, Yang P. Microrna 199a-5p induces apoptosis by targeting junb. Sci Rep. 2018;8:6699.
Esteves JV, Yonamine CY, Pinto-Junior DC, Gerlinger-Romero F, Enguita FJ, Machado UF. Diabetes modulates micrornas 29b-3p, 29c-3p, 199a-5p and 532-3p expression in muscle: possible role in glut4 and hk2 repression. Front Endocrinol. 2018;9:536.
Davoli R, Gaffo E, Zappaterra M, Bortoluzzi S. Identification of differentially expressed small rnas and prediction of target genes in italian large white pigs with divergent backfat deposition. Anim Genet. 2018;49:205–14.
Kajimoto K, Naraba H, Iwai N. Microrna and 3t3-l1 pre-adipocyte differentiation. RNA. 2006;12:1626–32.
Laine SK, Alm JJ, Virtanen SP, Aro HT, Laitala-Leinonen TK. Micrornas mir-96, mir-124, and mir-199a regulate gene expression in human bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2012;113:2687–95.
Watanabe T, Sato T, Amano T, Kawamura Y, Kawamura N, Kawaguchi H, et al. Dnm3os, a non-coding rna, is required for normal growth and skeletal development in mice. Dev Dyn. 2008;237:3738–48.
Aranda JF, Rathjen S, Johannes L, Fernández-Hernando C. MicroRNA 199a-5p attenuates retrograde transport and protects against toxin-induced inhibition of protein biosynthesis. 2018, 2018;38(11).
Aranda JF, Canfrán-Duque A, Goedeke L, Suárez Y, Fernández-Hernando C. The miR-199-dynamin regulatory axis controls receptor-mediated endocytosis. J Cell Sci. 2015;128(17):3197–209.
Shi XE, Li YF, Jia L, Ji HL, Song ZY, Cheng J, et al. Microrna-199a-5p affects porcine preadipocyte proliferation and differentiation. Int J Mol Sci. 2014;15:8526–38.
Zhang XN, Liu LF, Dou CY, Cheng PP, Liu L, Liu HR, et al. Ppar gamma-regulated microrna 199a-5p underlies bone marrow adiposity in aplastic anemia. Mol Ther Nucleic Acids. 2019;17:678–87.
Zhao YB, Zhao J, Zhang LJ, Shan RG, Sun ZZ, Wang K, et al. Microrna-370 protects against myocardial ischemia/reperfusion injury in mice following sevoflurane anesthetic preconditioning through plin5-dependent ppar signaling pathway. Biomed Pharmacother. 2019;113:108697.
Cui JX, Chen W, Zeng YQ. Development of fq-pcr method to determine the level of add1 expression in fatty and lean pigs. Genet Mol Res. 2015;14:13924–31.
Munoz M, Garcia-Casco JM, Caraballo C, Fernandez-Barroso MA, Sanchez-Esquiliche F, Gomez F, et al. Identification of candidate genes and regulatory factors underlying intramuscular fat content through longissimus dorsi transcriptome analyses in heavy iberian pigs. Front Genet. 2018;9:608.
Halmos T, Suba I. The physiological role of growth hormone and insulin-like growth factors. Orv Hetil. 2019;160:1774–83.
Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol. 2001;152:1057–70.
Wang C, Mei YJ, Li L, Mo DL, Li J, Zhang H, et al. Molecular characterization and expression analysis of caveolin-1 in pig tissues. Sci China C Life Sci. 2008;51:655–61.
Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–47.
Palacios-Ortega S, Varela-Guruceaga M, Algarabel M, Ignacio Milagro F, Alfredo Martinez J, de Miguel C. Effect of tnf-alpha on caveolin-1 expression and insulin signaling during adipocyte differentiation and in mature adipocytes. Cell Physiol Biochem. 2015;36:1499–516.
Frank PG, Pavlides S, Cheung MWC, Daumer K, Lisanti MP. Role of caveolin-1 in the regulation of lipoprotein metabolism. Am J Physiol Cell Physiol. 2008;295:C242–8.
Frank PG, Cheung MWC, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol. 2006;291:H677–86.
Ring A, Le Lay S, Pohl J, Verkade P, Stremmel W. Caveolin-1 is required for fatty acid translocase (fat/cd36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim Biophys Acta. 2006;1761:416–23.
Wang DX, Pan YQ, Liu B, Dai L. Cav-1 promotes atherosclerosis by activating jnk-associated signaling. Biochem Biophys Res Commun. 2018;503:513–20.
Li MZ, Wu HL, Luo ZG, **a YD, Guan JQ, Wang T, et al. An atlas of DNA methylomes in porcine adipose and muscle tissues. Nat Commun. 2012;3:850.
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. Tophat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36.
Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for rna-seq: accounting for selection bias. Genome Biol. 2010;11:R14.
Mao XZ, Cai T, Olyarchuk JG, Wei LP. Automated genome annotation and pathway identification using the kegg orthology (ko) as a controlled vocabulary. Bioinformatics. 2005;21:3787–93.
Liao BJ, Bao XC, Liu LQ, Feng SP, Zovoilis A, Liu WB, et al. Microrna cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem. 2011;286:17359–64.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(−delta delta c(t)) method. Methods. 2001;25:402–8.
Chen FF, **ong Y, Peng Y, Gao Y, Qin J, Chu GY, et al. Mir-425-5p inhibits differentiation and proliferation in porcine intramuscular preadipocytes. Int J Mol Sci. 2017;18:2101.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural Science Foundation of China (31601927), Fund for Distinguished Young Scholars from the Henan Academy of Agricultural Sciences (2019JQ05), and Financial Budget Project of Henan Province, grant number 182102110063, 2017-76-15. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
JW, YCP, and ABX designed the study. JW and FJC wrote the paper. HC collected the tissue samples. JW and YMC performed most of the experiments. FJC., QJZ, and LQR carried out part of the experiments. FJC carried out data analyses. SXB revised the manuscript. All authors contributed to drafting the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures involving animals described in the present study were performed according to the procedures of Institutional Animal Care and Use Committee (IACUC), approved by the Institute of Animal Science of Henan Academy of Agricultural Sciences (code 7 Aug 2017). The pigs were allowed access to feed and water ad libitum under normal conditions, and all efforts were made to minimize their suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationship that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Read map** to reference porcine genome (susScr3).
Additional file 2
: Table S2. Information of novel lncRNAs.
Additional file 3
: Table S3. Information of the differently expressed lncRNAs.
Additional file 4
: Table S4. The information of the primers used for real-time qPCR.
Additional file 5
: Figure S1. Correlation analysis of lncRNA expression in different samples.
Additional file 6
: Figure S2. Correlation analysis of lncRNA expression in different samples.
Additional file 7
: Figure S3. Validation of expression trends of ten randomly selected lncRNAs (five upregulated and five downregulated at 75 dpc) by qRT-PCR. Note: GAPDH used as reference genes, and fold changes calculated using 2-△△Ct method (mean ± SD, n = 3, * p < 0.05,** p < 0.01).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Chen, My., Chen, Jf. et al. LncRNA IMFlnc1 promotes porcine intramuscular adipocyte adipogenesis by sponging miR-199a-5p to up-regulate CAV-1. BMC Mol and Cell Biol 21, 77 (2020). https://doi.org/10.1186/s12860-020-00324-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12860-020-00324-8