Abstract
Aromatase, the key enzyme in estrogen biosynthesis, converts androstenedione to estrone and testosterone to estradiol. The enzyme is expressed in various tissues such as ovary, placenta, bone, brain, skin, and adipose tissue. Aromatase enzyme is encoded by a single gene CYP 19A1 and its expression is controlled by tissue-specific promoters. Aromatase mRNA is primarily transcribed from promoter I.4 in normal breast tissue and physiological levels of aromatase are found in breast adipose stromal fibroblasts. Under the conditions of breast cancer, as a result of the activation of a distinct set of aromatase promoters (I.3, II, and I.7) aromatase expression is enhanced leading to local overproduction of estrogen that promotes breast cancer. Aromatase is considered as a potential target for endocrine treatment of breast cancer but due to nonspecific reduction of aromatase activity in other tissues, aromatase inhibitors (AIs) are associated with undesirable side effects such as bone loss, and abnormal lipid metabolism. Inhibition of aromatase expression by inactivating breast tumor-specific aromatase promoters can selectively block estrogen production at the tumor site. Although several synthetic chemical compounds and nuclear receptor ligands are known to inhibit the activity of the tumor-specific aromatase promoters, further development of more specific and efficacious drugs without adverse effects is still warranted. Plants are rich in chemopreventive agents that have a great potential to be used in chemotherapy for hormone dependent breast cancer which could serve as a source for natural AIs. In this brief review, we summarize the studies on phytochemicals such as biochanin A, genistein, quercetin, isoliquiritigenin, resveratrol, and grape seed extracts related to their effect on the activation of breast cancer-associated aromatase promoters and discuss their aromatase inhibitory potential to be used as safer chemotherapeutic agents for specific hormone-dependent breast cancer.
Similar content being viewed by others
Background
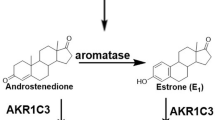
Aromatase is a member of the cytochrome P450 enzyme family and a product of the CYP 19A1 gene [1]. This membrane-bound protein (aromatase) is the rate limiting enzyme in the conversion of androstenedione to estrone (E1) and of testosterone to estradiol (E2) (Figure 1). Aromatase consists of two components: the hemoprotein aromatase cytochrome P450 encoded by the CYP19A1 gene and expressed only in steroidogenic cells, and the flavoprotein NADPH-cytochrome P450 reductase, expressed ubiquitously in many cell types [2–4]. The enzyme (aromatase) is localized in the endoplasmic reticulum of a cell, and catalyzes three hydroxylation reactions that convert androstenedione to E1 and testosterone to E2 [5, 6]. The enzyme activity is increased by alcohol, age, obesity, insulin and gonadotropins [7]. The CYP19A1 gene is highly expressed in the human placenta and in the granulosa cells of the ovarian follicles. However, many nonglandular tissues including liver, muscle, brain, bone, cartilage, blood vessels, breast (both normal and carcinogenic) and adipose tissues have lower level of CYP 19A1 expression under the control of tissue-specific promoters [8]. Inhibition of aromatase enzyme activity has been shown to reduce estrogen production throughout the body and aromatase inhibitors (AIs) are being used clinically to retard the development and progression of hormone-responsive breast cancer [6, 7].
The aromatase gene and tissue-specific promoter expression
Human aromatase is a 58 kDa protein which was first purified from placental microsomes in 1980s [9]. Only recently has the crystal structure of human placental aromatase been described [5]. Aromatase is encoded by a single copy of the CYP19A1 gene which is ~123 kb long, located on the short arm of the chromosome 15 (15q21), and is transcribed from the telomere to the centromere [2, 10–12]. The coding region spans 30 kb and includes nine translated exons (II-X) with two alternative polyadenylation sites [2]. The ATG translation initiation site is located on the exon II. There are a number of alternative non-coding first exons (I.1, I.2, I.3, I.4, I.5, I.6, I.7, and PII) which are expressed in tissue-specific manner, lie upstream to the coding region and are spliced to a common acceptor sites in exon 2 [13–68]. In addition, many natural products that have been used traditionally for nutritional or medicinal purposes as botanical dietary supplements (BDS) may also afford as AIs with reduced side effects [61, 69, 70]. Because many natural products are associated with low toxicity, they are potentially excellent candidates for use as chemopreventive agents [71–73]. Epidemiological evidence suggests that women living in Asia, where diets have traditionally included soybean products, report fewer postmenopausal symptoms and experience fewer breast cancers than women in Western countries [74–77]. More specifically, Asian women have a 3-fold lower breast cancer risk than women in the United States, independent of body weight [78]. Furthermore, serum concentrations of E2 are 40% lower in Asian women compared with their Caucasian counterparts [79]. Thus, environmental and dietary factors may explain at least some of the discrepancy in breast cancer risk between Asian and western populations [74, 75]. Despite the known AIs, there is still a need of searching for new AIs from natural products for future drug development [68]
Among the natural products tested as AIs, phytoestrogens, such as flavones and isoflavones are able to bind ER and induce estrogen action [77]. The binding characteristics and the structural requirements necessary for the inhibition of human aromatase by flavones and isoflavones were obtained by using computer modeling and confirmed by site-directed mutagenesis [80–82]. It was found that these compounds bind to the active site of aromatase in an orientation in which their rings A and C mimic rings D and C of the androgen substrate, respectively [80]. Until now ~ 300 natural products, most of them are phytoestrogens, have been evaluated for their ability to inhibit aromatase using noncellular (mostly using human microsome as a source of aromatase enzyme), cell-based, and in vivo aromatase inhibition assays [61, 83–85]; however, only a few studies (biochanin A from red clover, genistein from soybean, quercetin, isoliquiritigenin from licorice, resveratrol from grape peel and extracts of grape seeds, Figure 3) have been reported for their effect on aromatase promoter I.4, I.3/II activity [86–91]. The exact mechanisms how these plant products adapted to inhibit aromatase gene expression or enzyme activity is not fully understood.
Biochanin A (5, 7-dihydroxy-4'-methoxyisoflavone) is an isoflavone extracted from red clover (Trifolium pretense) by Pope et al. [92]. The first evidence that red clover has estrogenic activity were reported by Bennets et al. [93] after observing breeding problems of sheep grazing on red clover pastures which have been attributed to the isoflavone and coumestrol content of red clover. Serious fertility disturbances indicating estrogenic stimulation of cattle fed with red clover silage were reported [94–96]. Although biochanin A was moderately active in inhibiting microsomal aromatase activity (IC50: 5-10 μM) but was strongly active when tested in JEG-3 cells (human placental choriocarcinoma cell line). However, it did not inhibit aromatase activity in granulosa-luteal cells, and human preadipocyte cells and was also inactive in trout ovarian aromatase assay [61]. Interestingly, in MCF-7 cells (ER-positive breast cancer cells) biochanin A exhibited a dual action. It inhibited aromatase activity at low concentrations, but was estrogenic at high concentrations [97]. Furthermore, in SK-BR3 cells (ER-negative breast cancer cells) biochanin A was reported to inhibit aromatase enzyme activity and reduce mRNA expression. By using a luciferase reporter gene assay it was demonstrated that this phytochemical (biochanin A) was able to suppress the activation of breast-specific promoter I.3/II [88]. However, it is not known whether this inhibition is mediated through a PGE-2 or cAMP dependent PKA mechanisms. When genistein (a major metabolite of biochanin A) was tested in the same model, it was also found to suppress promoter I.3/II activation and showed an inhibition of aromatase enzyme activity [88]. Therefore, the inhibitory effect of biochanin A on aromatase promoter activation was suggested by the authors to be due to its metabolic conversion to genistein rather than its direct effect [88].
Genistein is a major phytoestrogen isolated from soybean, a potential nutraceutical, geared for women suffering from perimenopausal symptoms [98–101]. Genistein is also found in a number of other plants such as fava beans, lupin, kudzu, and psoralea [102]. Genistein is believed to be a chemopreventive agent against various types of cancers, including prostate, cervix, brain, breast, esophagus and colon [103]. Genistein was shown to increase aromatase activity in human adrenocortical carcinoma (H295R) cells and in isolated rat ovarian follicles [104, 105]. Dietary genistein, which produced circulating concentrations consistent with human exposures, did not act as an aromatase inhibitor; rather, dietary intake of genistein negated the inhibitory effect of an aromatase inhibitor letrozole (a 3rd generation aromatase inhibitor), by stimulating the growth of aromatase-expressing estrogen-dependent breast tumors [106]. This study raises concerns about the consumption of genistein-containing products by postmenopausal women with advanced breast cancer who may be treated with letrozole. Genistein suppressed promoter I.3/II transactivity in SK-BR-3 cells (an ER-negative breast cancer cell line), however, in HepG2 cells, genistein was found to induce promoter-specific aromatase mRNA expression with significant increases in promoters I.3 and II [89]. In addition, the phosphorylated forms of PKCα, p38, MEK and ERK1/2 kinases were also induced in HepG2 cells by genistein [89]. There are also some reports of a weak inhibition of aromatase enzyme activity by genistein as well [80, 107] and a decrease in the transcription of Cyp19 mRNA in human granulosa luteal cells [108].
Quercetin is one of the most abundant flavonols found in plants. Quercetin was found to inhibit human aromatase activity in placental microsomes [109]. When tested in cellular systems utilizing adrenocortical carcinoma cells, preadipocyte cells, or in co-culture experiments, it exhibited either a mild or no effect [86, 110, 111]. In the primary culture of human granulosa-luteal cells quercetin was able to reduce aromatase mRNA expression in a dose-dependent manner after an exposure period of 48 h [108]. In another study, H295R human adrenocortical carcinoma cells were exposed to quercetin for 24 h and an increase in aromatase enzyme activity was observed at lower concentration, while a decrease in the enzyme activity was observed at higher concentrations [105]. Quercetin increased p II and I.3-specific aromatase transcripts about 2.6-and 2-fold in H295R cells after 24 h exposure probably by enhancing intracellular cAMP levels [105].
Isoliquiritigenin, a flavonoid from licorice (Glycyrrhiza glabra), was found to be an inhibitor of aromatase enzyme activity in vitro [90]. Moreover, this compound was able to block MCF-7aro cells(MCF-7 cells stably transfected with CYP19) growth and when added in diet inhibited significantly the xenograft growth in ovariectomized athymic mice transplanted with MCF-7aro cells [90]. Isoliquiritigenin also inhibited aromatase mRNA expression and suppressed the activity of CYP19 promoters I.3 and II [90] in MCF-7 cells. Furthermore, binding of C/EBP to PII promoter of CYP19 was suppressed by isoliquiritigenin [90]. This study indicated that isoliquirititigenin has the potential to be used as a tissue-specific aromatase inhibitor in breast cancer.
The aromatase inhibitory activity of grapes and grape seed extracts (GSE) has been studied by many investigators [61, 83, 91]. The active chemicals found in grapes and red wine are procyanidin dimers that are also present in high concentrations in grape seeds [87]. GSE is composed of about 74-78% of proanthocyanidins and <6% of free flavanol monomers such as catechin, epicatechin, and their gallic acid esters [87]. Through the suppression of the expression of CREB-1 and glucocorticoid receptor (GR), grape seed extracts (GSE) has been found to decrease the expression of aromatase in MCF-7 and SK-BR-3 cells by suppressing the activity of promoters I.3/II, and I.4 in a dose-dependent manner [87]. The GSE (IH636) is in phase I clinical trials for the prevention of breast cancer in postmenopausal women who have an increased risk of breast cancer development [61].
The grape peel contains resveratrol, a polyphenolic compound which has structural similarity with estrogen [91]. This nonflavonoid phytoestrogen inhibited aromatase activity in MCF-7aro cells. In SK-BR-3 cells resveratrol significantly reduced aromatase mRNA and protein expression in a dose-dependent manner [91]. Moreover, this compound was able to repress the transactivation of CYP19 promoters I.3 and II in SK-BR-3 cells [91], which indicate that resveratrol could be able to reduce localized estrogen production in breast cancer cells.
Future directions
The expected direct outcome of aromatase inhibition is the maintenance of low levels of estrogen in the breast and surrounding adipose tissue. Understanding the molecular mechanism by which aromatase promoters I.4 and I.3/II are regulated is clinically significant and useful for develo** new drugs. Although only a few plant products have been documented to mediate their effects through aromatase promoters, there are many more potent natural products (such as white button mushroom (Agaricus bisporus) which is in phase I trials [83]) which could be potential candidates for future study. Moreover, accumulating evidence suggests that beside transcription factors and co-regulators there are many other factors such as cyclooxygenases (COX) which are involved in tissue-specific aromatase promoter regulation [112, 113]. Selective COX inhibitors from natural products can be used to suppress CYP19A1 gene expression. Studies also indicate that CYP 19A1 regulations are also under epigenetic control, including DNA methylation, and histone modification, which can add a new layer of complexity in the regulation of the aromatase gene [114]. DNA methylation generally occurs in gene promoters where the CpG rich dinucleotides are located. However, DNA methylation of CpG-poor promoter regions has also been shown as a mechanism of mediating tissue-specific gene transcription through the inhibition of transcription factor binding [115, 116]. Aromatase promoter I.3/II has six CpG dinucleotides subjected to methylation of cytosines and can be considered as CpG-poor promoter. However, in human skin fibroblasts hypermethylation of almost all six CpG sites resulted in markedly reduced aromatase promoter I.3/II activity, whereas hypomethylation of only two of the six sites led to increased promoter activity associated with an increase in cAMP [14]. In contrast to these studies, in breast adipose fibroblasts (BAF) promoter I.4 and I.3/II derived mRNA were not dependent on the CpG methylation status within respective aromatase promoters [114]. Further, DNA methylation is catalyzed by DNA methyl transferases (DNMTs). Inhibition of DNA methylation by 5-aza-2'-deoxycytidine, which is also a specific DNMT inhibitor, increased CYP19 mRNA expression in BAFs and breast cell lines [114]. These studies indicate that disruption in epigenetic regulation may give rise to increase in aromatase levels in the breast [114]. There are many synthetic chemicals that are undergoing clinical trials to be used as epigenetic drugs (epidrugs) for breast cancer treatment [117]. The major problems of these drugs are the unwanted side effects. Many natural products have the potential to be used as better epidrugs than synthetic epidrugs. One of the best examples is (-) - epigallocatechin-3-gallate from green tea which is used as demethylating agents for breast cancer patients [118–120]. Therefore extensive investigations in natural products seem promising or necessary.
Conclusions
Aromatase is a well-established molecular target and the AIs are proving to be an effective new class of agent for the chemoprevention of breast cancer. Regulation of aromatase expression in human tissues is a complex phenomenon, involving alternative promoter sites that provide tissue specific control. The promoters I.3 and II are the major promoters directing aromatase expression in breast cancer. The drugs that can selectively inhibit aromatase expression may be useful to obviate side effects induced by the nonselective AIs. Although many synthetic chemicals are used to inhibit tissue-specific inactivation of aromatase promoters I.3 and II, in the literature only a few natural products (we have included six of them) have been reported with such activities. More studies on natural products are necessary to find an appropriate tissue-specific AI.
Author's information
Shabana I. Khan is the Senior Scientist at the National Center for Natural Products Research and Associate Professor of the Department of Pharmacognosy at the University of Mississippi, University, MS 38677, USA. Jian** Zhao is the Associate Research Scientist at the National Center for Natural Products Research at the University of Mississippi, University, MS 38677, USA. Ikhlas A. Khan is the Assistant Director of the National Center for Natural Products Research and Professor of Pharmacognosy, School of Pharmacy of the University of Mississippi, University, MS 38677, USA. Larry A. Walker is the Director of the National Center for Natural Products Research at the University of Mississippi, and Associate Director for Basic Research Oxford, University of Mississippi Cancer Institute and the Professor of Pharmacology, School of Pharmacy of the University of Mississippi, University, MS 38677, USA, Asok K. Dasmahapatra is the Research Scientist at the National Center for Natural Products Research and Assistant Professor of the Department of Pharmacology, School of Pharmacy of the University of Mississippi, University, MS 38677, USA.
Abbreviations
- AIs:
-
Aromatase inhibitors
- COX:
-
Cyclooxygenase
- E1:
-
estrone
- E2:
-
17β- estradiol
- ER:
-
Estrogen receptor
- PGE:
-
prostaglandin
- PPAR:
-
Peroxisome proliferator activator receptor
- C/EBP:
-
CCAT/enhancer binding protein.
References
Simpson ER: Aromatase: biologic relevance of tissue specific expression. Semin Reprod Med. 2004, 22: 11-23.
Means GD, Mahendroo MS, Corbin CJ, Mathis JM, Powell FE, Mendelson CR, Simpson ER: Structural analysis of the gene encoding human aromatase cytochrome P-450, the enzyme responsible for estrogen biosynthesis. J Biol Chem. 1989, 264: 19385-19391.
Zhao Y, Agarwal VR, Mendelson CR, Simpson ER: Transcriptional regulation of CYP 19 gene (aromatase) expression in adipose stromal cells in primary culture. J Steroid Biochem Mol Biol. 1997, 61: 203-210. 10.1016/S0960-0760(97)80013-1.
Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M: Aromatase-a brief overview. Annu Rev Physiol. 2002, 64: 93-127. 10.1146/annurev.physiol.64.081601.142703.
Ghosh D, Griswold J, Erman M, Pangborn W: Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009, 457 (7226): 219-223. 10.1038/nature07614.
Milani M, Jha G, Potter DA: Anastrozole use in early stage breast cancer of post-menopausal women. Clin Med Ther. 2009, 1: 141-156.
Smith IE, Dowsett M: Aromatase inhibitors in breast cancer. N Engl J Med. 2003, 348 (24): 2431-2442. 10.1056/NEJMra023246.
Simpson ER: Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003, 86 (3-5): 225-230. 10.1016/S0960-0760(03)00360-1.
Czajka-Oraniec I, Simpson ER: Aromatase research and its clinical significance. Endokrynol Pol. 2010, 61: 126-134.
Harada N, Yamada K, Saito K, Kibe N, Dohmae S, Takagi Y: Structural characterization of the human estrogen synthetase (aromatase) gene. Biochem Biophys Res Commun. 1990, 166: 365-372. 10.1016/0006-291X(90)91954-Q.
Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW: P450 superfamily: update on new sequences, gene map**, accession numbers and nomenclature. Pharmacogenetics. 1996, 6: 1-42. 10.1097/00008571-199602000-00002.
Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S: Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005, 57: 359-383. 10.1124/pr.57.3.6.
Bulun SE, Simpson ER: Aromatase expression in women's cancer. Adv Exp Med Biol. 2008, 630: 112-132. 10.1007/978-0-387-78818-0_8.
Chen D, Reierstad S, Lu M, Lin Z, Ishikawa H, Bulun SE: Regulation of breast cancer-associated aromatase promoters. Cancer Lett. 2009, 15-27. 273
Jiao J, **ang H, Liao Q: Recent advancement in nonsteroidal aromatase inhibitors for treatment of estrogen-dependent breast cancer. Curr Med Chem. 2010, 17: 3476-3487. 10.2174/092986710792927877.
Mahendroo MS, Means GD, Mendelson CR, Simpson ER: Tissue-specific expression of human P-450AROM. The promoter responsible for expression in adipose tissue is different from that utilized in placenta. J Biol Chem. 1991, 266: 11276-11281.
Simpson ER, Michael MD, Agarwal VR, Hinshelwood MM, Bulun SE, Zhao Y: Expression of the CYP19 (aromatase) gene: an unsual case of alternative promoter usage. FASEB J. 1997, 11: 29-36.
Sebastian S, Takayama K, Shozu M, Bulun SE: Cloning and characterization of a novel endothelial promoter of the human CYP19 (aromatase P450) gene that is up-regulated in breast cancer tissue. Mol Endocrinol. 2002, 16: 2243-2254. 10.1210/me.2002-0123.
Bulun SE, Takayama K, Suzuki T, Sasano H, Yilmaz B, Sebastian S: Organization of the human aromatase p450 (CYP19) gene. Semin Reprod Med. 2004, 5-9. 22
Zhao Y, Mendelson CR, Simpson ER: Characterization of the sequences of human CYP19 (aromatase) gene that mediate regulation by glucocorticoids in adipose stomal cells and fetal hepatocytes. Mol Endocrinol. 1995, 9: 340-349. 10.1210/me.9.3.340.
Zhao Y, Nichols JE, Bulun SE, Mendelson CR, Simpson ER: Aromatase p450 gene expression in human adipose tissue. Role of Jak/STAT pathway in regulation of the adipose-specific promoter. J Biol chem. 1995, 270: 16449-16457. 10.1074/jbc.270.27.16449.
Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S, Chen D: Regulation of aromatase expression in breast cancer tissue. Ann NY Acad Sci. 2009, 1155: 121-131. 10.1111/j.1749-6632.2009.03705.x.
Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE: Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2001, 61: 2328-2334.
Utsumi T, Harada N, Maruta M, Takagi Y: Presence of alternatively spliced transcripts of aromatase gene in human breast cancer. J Clin Endocrinol Metab. 1996, 81: 2344-2349. 10.1210/jc.81.6.2344.
Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER: Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab. 1996, 81: 3843-3849. 10.1210/jc.81.11.3843.
American Cancer Society: Cancer statistics. 2006, [http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/index]
IARC (International Agency of Research on Cancer): Overall evaluation of Carcinogenicity: An updating of IARC Monographs Volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987, 7: 272-310.
IARC (International Agency for Research on Cancer): Hormonal concentration and postmenopausal hormone therapy. IARC Monogr Eval Carcinog Risks Hum. 1999, 72: 474-530.
Cavalieri E, chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T: Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006, 1766: 63-78.
Yager JD, Davidson NE: Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006, 354: 270-282. 10.1056/NEJMra050776.
Longcope C, Pratt JH, Schneider SH, Fineberg SE: Aromatization of androgens by muscle and adipose tissue in vivo. J Clin Endocrinol Metab. 1978, 46: 146-152. 10.1210/jcem-46-1-146.
Sasano H, Uzuki M, Sawai T, Nagura H, Matsunaga G, Kashimoto O, Harada N: Aromatase in human bone tissue. J Bone Miner Res. 1997, 12: 1416-1423. 10.1359/jbmr.1997.12.9.1416.
Subramanian A, Salhab M, Mokbel K: Oestrogen producing enzymes and mammary carcinogenesis: a review. Breast Cancer Res Treat. 2008, 111: 191-202. 10.1007/s10549-007-9788-0.
Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y: Localized expression of aromatase in human vascular tissues. Cir Res. 1999, 84: 1285-1291.
Simpson ER, Davis SR: Minireview: aromatase and the regulation of estrogen biosynthesis-some new perspectives. Endocrinology. 2001, 142: 4589-4594. 10.1210/en.142.11.4589.
Castagnetta LA, Lo Casto M, Granata OM, Polito L, Calabro M, Lo Bue A, Bellavia V, Carruba G: Estrogen content and metabolism in human breast tumor tissues and cells. Ann NY Acad Sci. 1996, 784: 314-324. 10.1111/j.1749-6632.1996.tb16246.x.
Miki Y, Suzuki T, Sasano H: Controversies of aromatase localization in human breast cancer-stromal versus parenchymal cells. J Steroid Biochem Mol Biol. 1990, 37: 1055-1059. 10.1016/0960-0760(90)90465-W.
Suzuki T, Miki Y, Akahira J-I, Moriya T, Ohuchi N, Sasano H: Aromatase in human breast carcinoma as a key regulator of intratumoral sex steroid concentrations. Endocrine J. 2008, 55: 455-463. 10.1507/endocrj.K07E-053.
Miller WR, Anderson TJ, Jack WJ: Relationship between tumor aromatase acitivity, tumor characteristics and response to therapy. J steroid Biochem Mol Biol. 1990, 37: 1055-1059. 10.1016/0960-0760(90)90465-W.
Suzuki T, Moriya T, Ishida T, Ohuchi N, Sasano H: Intracrine mechanism of estrogen synthesis in breast cancer. Biomed Pharmacother. 2003, 57: 460-462. 10.1016/j.biopha.2003.09.007.
Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR: Immunohistochemistry of estrogen and projesteron receptors reconsidered: experience with 5,993 breast cancers. Am J Clin pathol. 2005, 123: 21-27. 10.1309/4WV79N2GHJ3X1841.
Wang T, You Q, Huang FS, **ang H: Recent advances in selective estrogen receptor modulators for breast cancer. Mini Rev Med Chem. 2009, 9: 1191-1201. 10.2174/138955709789055207.
Brueggemeier RW, Hackett JC, Diaz-Cruz ES: Aromatase inhibitors in the treatment of breast cancer. Endocr Rev. 2005, 26: 331-345. 10.1210/er.2004-0015.
Nabholtz JM: Long-term safety of aromatase inhibitors in the treatment of breast cancer. Ther Clin Risk Manag. 2008, 4: 189-204.
Edwards BJ, Raisch DW, Shankaran V, McKoy JM, Gradishar W, Bunta AD, Samaras AT, Boyle SN, Bennett CL, West DP, Guise TA: Cancer therapy associated bone loss: implications for hip fractures in mid-life women with breast cancer. Clin cancer Res. 2011, 17: 560-568. 10.1158/1078-0432.CCR-10-1595.
Mazziotti G, Canalis E, Giustina A: Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med. 2010, 123: 877-884. 10.1016/j.amjmed.2010.02.028.
Murata Y, Ogawa Y, Saibara T, Nishioka A, Fujiwara Y, Fukumoto M, Inomata T, Enzan H, Onishi S, Yoshida S: Unrecognized hepatic steatosis and non-alcoholic steatohepatitis in adjuvant tamoxifen for breast cancer patients. Oncol Rep. 2000, 7: 1299-1304.
Pinto HC, Baptista A, Camilo ME, de Costa EB, Valente A, de Moura MC: Tamoxifen-associated steatohepatitis report of three cases. J Hepatol. 1995, 23: 95-97. 10.1016/0168-8278(95)80316-5.
Oien KA, Moffat D, Curry GW, Dickson J, Habeshaw T, Mills PR, MacSween RN: Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet. 1999, 353: 36-37. 10.1016/S0140-6736(05)74872-8.
Simpson E, Davis S: Why do the clinical sequelae of estrogen deficiency affect women more than men?. J Clin Endocrinol Metab. 1998, 83: 2214-10.1210/jc.83.6.2214.
Simpson E, Rubin G, Clyne C, Robertson K, Donnell L, Jones M, Davis S: The role of local estrogen biosynthesis in the males and females. Trends Endocrinol Metab. 2000, 11: 184-188. 10.1016/S1043-2760(00)00254-X.
Safi R, Kovacic A, Gaillard S, Mutara ER, Simpson ER, McDonnell DP, Clyne CD: Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor gamma coactivator-1 aplha on aromatase promoter II and its inhibition by activated retinoid × receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer Res. 2005, 65: 11762-11770. 10.1158/0008-5472.CAN-05-2792.
Rubin GL, Duong JH, Clyne CD, Speed CJ, Murata Y, Gong C, Simpson ER: Ligands of the peroxisomal proliferator-activated receptor gamma and the retinoid × receptor inhibit aromatase cytochrome P450 (CYP19) expression mediated by promoter II in human breast adipose. Endocrinology. 2002, 143: , 2863-2871.
Lebovitz HE: Differentiating members of the thiazolidinedione class: a focus on safety. Diabetes Metab Res Rev. 2002, 18 (suppl 2): S23-S29.
Sharma R, Sharma R, Verma U, Bhat NK: Novel drugs targeting retinoic acid receptors. JK Science. 2005, 7: 1-2.
Deb S, Zhou J, Amin SA, Imir AG, Yimaz MB, Lin Z, Bulun SE: A novel role of sodium butyrate in the regulation of cancer-associated aromatase promoters I.3 and II by disrupting transcriptional complex in breast adipose fibroblasts. J Biol Chem. 2006, 281: 2585-2597.
Weidle UH, Grossmann A: Inhibition of histone deacetylases: a new strategy to target epigenetic modifications of anticancer treatment. Anticancer Res. 2000, 20: 1471-1485.
Chen D, Reierstad S, Lin Z, Lu M, Brooks C, Li N, Innes J, Bulun SE: Prostaglandin E (2) induces breast cancer related aromatase promoters via activation of p38 and c-jun NH(2)-terminal kinase in adipose fibroblast. Cancer Res. 2007, 67: 8914-8922. 10.1158/0008-5472.CAN-06-4751.
Gore SD, Carducci MA: Modifying histones to tame cancer: clinical development of sodium phenylbutyrate and other histone deacetylase inhibitors. Expert Opin Investig drugs. 2006, 15: 721-727. 10.1517/13543784.15.6.721.
Braddock M, Murray C: 10th anniversary inflammation and immune diseases Drug Discovery and Development Summit. 20-21 March 2006, New Brunswick, USA. Expert Opin Investig Drugs. 2006, 15: 721-727. 10.1517/13543784.15.6.721.
Balunas MJ, Su B, Brueggemeier RW, Kinghorn AD: Natural products as aromatase inhibitors. Anticancer Agents Med Chem. 2008, 8: 646-682.
Daly JW: Marine toxins and nonmarine toxins: convergence or symbiotic organisms. J Nat Prod. 2004, 67: 1211-1215. 10.1021/np040016t.
Cardy J, Walsh C: Lessons from natural molecules. Nature. 2004, 432: 829-837. 10.1038/nature03194.
Butler MS: Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008, 25: 475-516. 10.1039/b514294f.
Newman DJ, Cragg GM: Microbial antitumor drugs: natural products of microbial origin as anticancer agents. Curr Opin Investig Drugs. 2009, 10: 1280-1296.
Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE: The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci. 2010, 31: 255-265. 10.1016/j.tips.2010.02.005.
Cragg GM, Grothaus PG, Newman DJ: Impact of natural products on develo** new anti-cancer agents. Chem Rev. 2009, 109: 3012-3042. 10.1021/cr900019j.
Eisenberg DM, Harris ES, Littlefield BA, Cao S, Craycroft JA, Scholten R, Bayliss P, Fu Y, Wang W, Qiao Y, Zhao Z, Chen H, Liu Y, Kaptchuk T, Hahn WC, Wang X, Roberts T, Shamu CE, Clardy J: Develo** a library of authenticated Traditional Chinese medicinal (TCM) plants for systematic biological evaluation-rationale, methods and preliminary results from a Sino-American collaboration. Fitoterapia. 2011, 82: 17-33. 10.1016/j.fitote.2010.11.017.
Basly JP, Lavier MC: Dietary phytoestrogens: potential selective estrogen enzyme modulators?. Planta Med. 2005, 71: 287-294. 10.1055/s-2005-864092.
Edmunds KM, Holloway AC, Crankshaw DJ, Agarwal SK, Foster WG: The effects of dietary phytoestrogens on aromatase activity in human endometrial stromal cells. Reprod Nutr Dev. 2005, 45: 709-720. 10.1051/rnd:2005055.
Whitehead SA, Lacey M: Phytoestrogens inhibit aromatase but not 17β-hydroxysteroid dehydrogenase (HSD) type 1 in human granulosa-luteal cells: evidence for FSH induction of 17β-HSD. Human Repro. 2003, 18: 487-494. 10.1093/humrep/deg125.
Rice S, Whitehead SA: Phytoestrogens and breast cancer-promoters or protectors?. Endocrine-Related Cancer. 2006, 13: 995-1015. 10.1677/erc.1.01159.
Bal Z, Gust R: Breast cancer, estrogen receptor and ligands. Arch Pharm Chem Life Sci. 2009, 342: 133-149. 10.1002/ardp.200800174.
Adlercreutz H: Epidemiology of phytoestrogens. Bailliers Clin Endocrinol Metab. 1998, 12: 605-623. 10.1016/S0950-351X(98)80007-4.
Nichenametla SN, Taruscio TG, Barney DL, Exon JH: A review of the effects of mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006, 46: 161-183. 10.1080/10408390591000541.
Usui T: Pharmaceutical prospects of phytoestrogens. Endocrine J. 2006, 53: 7-20. 10.1507/endocrj.53.7.
Mense SM, Hei TK, Ganju RK, Bhat HK: Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environ Health Persp. 2008, 166: 426-433.
Ursin G, Bernstein L, Pike MG: Breast cancer. Cancer Surv. 1994, 19-20: 241-264.
Peeters PH, Keinan-Boker L, van der Schouw YT, Grobbee DE: Phytoestrogens and breast cancer risk. Review of the epidemiological evidence. Breast cancer Res Treat. 2003, 77: 171-183. 10.1023/A:1021381101632.
Kao YC, Zhou C, Sherman M, Laughton CA, Chen S: A site-directed mutagenesis study Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens. Environ Health Perspect. 1998, 106: 85-92.
Chen S, Zhang F, Sherman MA, Kijima I, Cho M, Yuan YC, Toma Y, Osawa Y, Zhou D, Eng ET: Structure-function studies of aromatase and its inhibitors: a progress report. J Steroid Biochem Mol Biol. 2003, 86: 231-237. 10.1016/S0960-0760(03)00361-3.
Paoletta S, Steventon GB, Wildeboer D, Ehrman TM, Hylands PJ, Barlow DJ: Screening of herbal constituents for aromatase inhibitory activity. Bioorganic Med Chem. 2008, 16: 8466-8470. 10.1016/j.bmc.2008.08.034.
Adam S, Chen S: Phytochemicals for breast cancer prevention by targeting aromatase. Front Biosci. 2009, 14: 3846-3863.
Zhao J, Dasmahapatra AK, Khan SI, Khan IA: Anti-aromatase activity of the constituents from damiana (Turnera diffusa). J Ethnopharmacol. 2008, 120: 387-393. 10.1016/j.jep.2008.09.016.
Balunas MC, Kinghorn AD: Natural compounds with aromatase inhibitory activity: an update. Planta Med. 2010, 76: 1087-1093. 10.1055/s-0030-1250169.
Sanderson JT, Hordijk J, Denison MS, Springsteel MF, Nantz MH, van den Berg M: Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2004, 82: 70-79. 10.1093/toxsci/kfh257.
Kijima I, Phung S, Hur G, Kwok SL, Chen S: Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Res. 2006, 5960-5967. 66
Wang Y, Gho WM, Chan FL, Chen S, Leung LK: The red clover (trifolium pretense) isoflavone biochenin A inhibits aromatase activity and expression. Brit J Nutr. 2008, 99: 303-310.
Ye L, Chan MY, Leung LK: The soy isoflavone genistein induces estrogen synthesis in the extragonadal pathway. Mol Cell Endocrionol. 2009, 73-80. 302
Ye L, Gho WM, Chan FL, Chen S, Leung LK: Dietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overespressing aromatase. Int J can. 2009, 124: 1028-1036. 10.1002/ijc.24046.
Wang Y, Lee KW, Chan FL, Chen S, Leung LK: The red wine polyphenol reseveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol Sci. 2006, 92: 71-77. 10.1093/toxsci/kfj190.
Pope GS, Elcoate PV, Simpson SA, Andrews DG: Isolation of an oestrogenic isoflavone (biochanin A) from red clover. Chem Indus. 1953, 1092
Bennets HW, Underwood EJ, Shier FL: A specific breeding problem of sheep on subterranean clover pastures in western Australia. Aust Vet J. 1946, 22: 2-12. 10.1111/j.1751-0813.1946.tb15473.x.
Linder HR: Occurrence of anabolic agents in plants and their importance. Environ Qual saf Suppl. 1976, 5: 151-158.
Kallela K, Heinonen K, saloniemi H: Plant oestrogens; the cause of decresed fertility in cows. A case report. Nord Vet Med. 1984, 36: 124-129.
Saloniemi H, Wahala K, Nykanen-Kurki P, Kallela K, Saastamoinen I: Phytoestrogen content and estrogenic effect of legume fodder. Proc Soc Exp Biol Med. 1995, 208: 13-17.
Almstrup K, Fernandez MF, Petersen JH, Olea N, Skakkebaek NE, Leffers H: Dual effects of phytoestrogens results in u-shaped dose-response curves. Environ Health perspect. 2002, 110: 743-748. 10.1289/ehp.02110743.
Franke AA, Custer LJ: High-performance liquid chromatographic assay of isoflavonoids and coumestrol from human urine. J chromatogram B Biomed Appl. 1994, 662: 47-60. 10.1016/0378-4347(94)00390-4.
Mazur W, Fotsis T, Wahala K, Ojala S, Salakka A, Adlercreutz H: Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal Biochem. 1996, 233: 169-180. 10.1006/abio.1996.0025.
Nestel PJ, Yamashita T, sasahara T, Pomeroy S, Dart A, Komesaroff P, Owen A, Abbey M: Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler thromb Vasc Biol. 1997, 17: 3392-3398. 10.1161/01.ATV.17.12.3392.
Manonai J, Songchitsomboon S, Chanda K, Hong JH, Komindr S: The effect of a soy-rich diet on urogenital atrophy: a randomized, cross-over trial. Maturitas. 2006, 54: 135-140. 10.1016/j.maturitas.2005.09.011.
Meeran SM, Ahmad A, Tollefsbol TO: Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics. 2010, 1: 101-116. 10.1007/s13148-010-0011-5.
Barnes S: Effect of genistein on in vitro and in vivo model of cancer. J nutr. 1995, 125: 777s-783s.
Myllymaki S, Haavisto T, Vainio M, Toppari J, Paranko J: In vitro effects of diethylstilbestrol, genistein, 4-tert-butylphenol, and 4-tert-octylphenol on steroidogenic activity of isolated mature rat ovarian follicles. Toxicol Appl Pharmacol. 2005, 204: 69-80. 10.1016/j.taap.2004.08.009.
Sanderson JT, Hordijk J, Denison MS, Springsteel MF, Nantz MH, van der Berg M: Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2004, 82: 70-79. 10.1093/toxsci/kfh257.
Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG: Dietary genistein negates the inhibitory effect of latrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008, 29: 2162-2168. 10.1093/carcin/bgn161.
Pelissero C, Lenczowski MJ, Chinzi D, Davail-Cuisset B, Sumpter JP, Fostier A: Eflavonoids on aromatase activity, an in vitro study. J steroid Biochem Mol boil. 1996, 57: 215-223. 10.1016/0960-0760(95)00261-8.
Rice S, Mason HD, Whitehead SA: Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol. 2006, 101: 216-225. 10.1016/j.jsbmb.2006.06.021.
Kellis JT, Vickery LE: Inhibition of human estrogen synthase (aromatase) by flavones. Science. 1984, 225: 1032-1034. 10.1126/science.6474163.
Wang C, Makela T, hase T, Kurzer MS: Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J steroid Biochem Mol Biol. 1994, 50: 205-212. 10.1016/0960-0760(94)90030-2.
van Meeuwen JA, Korthagen PC, de Jong PC, Piersma AH, van den Berg M: (Anti)estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture. Toxicol appl Pharmacol. 2007, 221: 372-383. 10.1016/j.taap.2007.03.016.
Prosperi JR, Robertson FM: Cyclooxygenase-2 directly regulates gene expression of P450 Cyp19 aromatase promoter regions pII, p1.3, and pI.7 and estradiol production in human breast tumor cells. Prostaglandins Other Lipid Mediat. 2006, 81: 55-70. 10.1016/j.prostaglandins.2006.07.003.
Brueggemeier RW, Su B, Darby MV, Sugimoto Y: Selective regulation of aromatase expression for drug discovery. J Steroid Biochem Mol Biol. 2010, 118: 207-210. 10.1016/j.jsbmb.2009.11.009.
Knower KC, To SQ, Simpson ER, Clyne CD: Epigenetic mechanisms resulting CYP19 transcription in human breast adipose fibroblasts. Mol Cell Endocrinol. 2010, 321: 123-130. 10.1016/j.mce.2010.02.035.
Fujii G, nakamura Y, Tsukamoto D, Ito M, Shiba T, Takamatsu N: CpG methylation at the USF-binding site is important for the liver-specific transcription of the chipmunk HP-27 gene. Biochem J. 2006, 395: 203-209. 10.1042/BJ20051802.
Jones B, Chen J: Inhibition of INF-gamma transcription by site-specific methylation during T helper cell development. EMBO J. 2006, 25: 2443-2452. 10.1038/sj.emboj.7601148.
Miyamoto K, Ushijima T: Diagnostic and therapeutic applications of epigenetics. Jap J Clin Oncol. 2005, 35: 293-301. 10.1093/jjco/hyi088.
Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS: Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63: 7563-7570.
Moyers SB, Kumar NB: Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr Rev. 2004, 62: 204-211. 10.1111/j.1753-4887.2004.tb00041.x.
Krik H, Cefalu WT, Ribnicky D, Liu Z, Eilertsen KJ: Botanicals as epigenetic modulators for mechanisms contributing to development of metabolic syndrome. Metab Clinic Exp. 2008, 57 (suppl 1): S16-S23.
Acknowledgements
United States Department of Agriculture (USDA), Agriculture Research Service Specific Cooperative Agreement No 58-6408-2-0009 is acknowledged for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SIK, JZ, IAK, LAW and AKD are contributed in literature review, graphics work and writing the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Khan, S.I., Zhao, J., Khan, I.A. et al. Potential utility of natural products as regulators of breast cancer-associated aromatase promoters. Reprod Biol Endocrinol 9, 91 (2011). https://doi.org/10.1186/1477-7827-9-91
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-9-91