Abstract
Background
Contrast-induced nephropathy (CIN) is the third leading cause of hospital-acquired acute kidney injury, and it is associated with poor long-term clinical outcomes. Although systolic heart failure is a well-known risk factor for CIN, no studies have yet evaluated the association between diastolic dysfunction and CIN.
Methods
We conducted a retrospective study of 735 patients who underwent percutaneous transluminal coronary angioplasty (PTCA) and had an echocardiography performed within one month of the procedure at our institute, between January 2009 and December 2010. CIN was defined as an increase of ≥ 0.5 mg/dL or ≥ 25% in serum creatinine level during the 72 hours following PTCA.
Results
CIN occurred in 64 patients (8.7%). Patients with CIN were older, had more comorbidities, and had an intra-aortic balloon pump (IABP) placed more frequently during PTCA than patients without CIN. They showed greater high-sensitivity C-reactive protein (hs-CRP) levels and lower estimated glomerular filtration rates (eGFR). Echocardiographic findings revealed lower ejection fraction and higher left atrial volume index and E/E’ in the CIN group compared with non-CIN group. When patients were classified into 3 groups according to the E/E’ values of 8 and 15, CIN occurred in 42 (21.6%) patients in the highest tertile compared with 20 (4.0%) in the middle and 2 (4.3%) in the lowest tertile (p < 0.001). In multivariate logistic regression analysis, E/E’ > 15 was identified as an independent risk factor for the development of CIN after adjustment for age, diabetes, dose of contrast media, IABP use, eGFR, hs-CRP, and echocardiographic parameters [odds ratio (OR) 2.579, 95% confidence interval (CI) 1.082-5.964, p = 0.035]. In addition, the area under the receiver operating characteristic curve of E/E’ was 0.751 (95% CI 0.684-0.819, p < 0.001), which was comparable to that of ejection fraction and left atrial volume index (0.739 and 0.656, respectively, p < 0.001).
Conclusions
This study demonstrated that, among echocardiographic variables, E/E' was an independent predictor of CIN. This in turn suggests that diastolic dysfunction may be a useful parameter in CIN risk stratification.
Similar content being viewed by others
Background
Contrast-induced nephropathy (CIN) is one of the principal complications which develop after procedures using contrast media (CM). Although there are some differences between institutions, the most commonly used definition of CIN in clinical trials is a rise in serum creatinine of > 0.5 mg/dL or > 25% from the baseline value within 72 hours after exposure to the CM. The incidence of CIN is reported to be about 2% in the general population, 7-15% in patients undergoing percutaneous coronary intervention, whereas it increases up to 50% in high-risk patients with diabetes (DM) and renal failure [1, 2]. Recently, risk stratification and application of preventive methods against CIN further reduced the incidence of CIN. However, CIN is still an important problem in that dependence for medical procedures using CM is steadily increasing and that it is closely related with poor long-term clinical outcomes: longer hospital stay, and increased mortality and post-procedural cardiovascular complications [3, 4].
Accordingly, many investigators have tried to reveal the pathogenesis of CIN. Delineated mechanisms include intra-renal vasoconstriction, reduced renal blood flow, medullary hypoxia, oxidative stress, inflammation, endothelial dysfunction, and direct tubular-epithelial cell injury by CM [5]. At the same time, risk factors potentiating the processes explained above were proved out: renal failure, old age, DM, congestive heart failure (CHF), hypotensive event, use of intra-aortic balloon pump (IABP), and ionic/ high-osmolar CM [6].
CHF, especially in advanced stages of New York Heart Association (NYHA) class 3–4 contributes to the development of CIN primarily by decreasing renal perfusion. It is related with systolic dysfunction and low stroke volume [7]. In fact, some researches specifically pointed ejection fraction (EF) < 30-40% out as an independent predictor of CIN [8]. However, CHF per se and just a past history of heart failure increased the incidence of CIN, irrespective of EF [9, 10]. Nevertheless, no studies have yet evaluated the association between diastolic dysfunction and CIN.
The golden standard estimating diastolic function of the heart is to measure left ventricular end-diastolic pressure (LVEDP) with catheterization. However, it is invasive and not a routine practice. On the other hand, E/E’ can be assessed non-invasively with echocardiography, and is known to be less influenced by heart rate, atrial activity, or ejection fraction [11]. In addition, E/E’ levels were well correlated with LVEDP, when patients were categorized into 3 groups based on the E/E’ values of 8 and 15 [12]. E/E’ > 11 also predicted LVEDP > 15 mmHg with a sensitivity of 75% and a specificity of 93% [13]. Among various doppler estimates of diastolic function such as E, E/A, decrease in E/A with the valsalva maneuver, deceleration time (DT), and pulmonary venous atrial reversal duration, E/E’ showed the highest predictive power for LVEDP [12].
So, we aimed to investigate whether E/E’ is an independent risk factor predicting the development of CIN. Effects of E/E’ and CIN on patient mortality were also evaluated.
Methods
Ethics statement
This study was carried out in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Yonsei University Health System Clinical Trial Center. Written consents were not required because this was a retrospective medical record-based study and personally identifiable information was anonymized.
Patients
This retrospective study included 735 patients who underwent percutaneous transluminal coronary angioplasty (PTCA) at Severance Hospital in Seoul, Korea from January 2009 to December 2010. PTCA was conducted in 6837 patients for the following reasons: 1) regular follow-up for known coronary artery occlusive disease (CAOD), 2) suspicion for CAOD based on clinical symptoms or study results (treadmill test, technetium sestamibi scan, or cardiac CT or MRI), or 3) to evaluate whether idiopathic CHF was due to ischemia. Among these, 3302 subjects who had undergone echocardiography within 1 month of PTCA were included. Exposure to other CM within 7 days of PTCA (n = 1576), an absence of data on serum creatinine during the 72 hours following the procedure (n = 503), end-stage renal disease (n = 408), underlying malignancy (n = 43), acute infection (n = 28), or age less than 18 years (n = 9) resulted in the exclusion of an additional 2567 patients.
All patients were hydrated with 0.9% saline at a rate of 1.0 mL/kg/hour for 12 hours pre- and post-exposure to the CM, according to the guidelines of our clinic. In case of an emergency procedure, hydration was initiated immediately prior to the start of angiography and continued for 24 hours after PTCA. Infusion rate was reduced to 0.5 mL/kg/hour when pulmonary edema was noted or ejection fraction was less than 30%.
Non-ionic, dimeric, and iso-osmolar contrast agent (iodixanol: Visipaque, GE Healthcare, Amersham, United Kingdom) was used for PTCA. A hypotensive event was defined as systolic blood pressure < 80 mmHg for at least 1 hour requiring inotropic therapy or IABP insertion within 24 hours of procedure, as described by Mehran et al. [14].
Data collection
Demographic and clinical data at the time of the PTCA were collected through medical chart review. Laboratory findings which were reported 24 hours prior to the PTCA and measured in overnight fasting status, were set as baseline values. CIN was defined as an increase of ≥ 0.5 mg/dL or ≥ 25% in serum creatinine level during the 72 hours following PTCA, that could not be better explained by alternative etiologies. We also used a second definition of CIN according to the Acute Kidney Injury Network (AKIN) criteria: a rise in serum creatinine ≥ 0.3 mg/dL within 48 hours of procedure [3, 15]. Oliguria was not considered because all patients were hydrated and some of them used diuretics.
Hemoglobin, albumin, blood urea nitrogen, creatinine, serum cholesterol, triglycerides, and glucose levels were measured by an Advia 2120 Hematology Analyzer (Siemens Health-care Diagnostics, Deerfield, Illinois). High-sensitivity C-reactive protein (hs-CRP) levels were measured with a BN II analyzer (Dade Behring, Newark, DE, USA) using the latex-enhanced immunonephelometric method. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation [16].
Cardiac status and echocardiographic parameters
Records of PTCA were reviewed for the urgency of the procedure, amount of CM used, interventional strategy, and the number of coronary arteries involved. We also investigated whether any patients had undergone coronary artery bypass graft surgery previously.
Echocardiography was performed with a SONOS 7500 (Philips Ultrasound, Bothell, WA, USA) according to the recommendations of the American Society of Echocardiography (ASE). Inter-ventricular septal thickness (IVSs, IVSd), posterior wall thickness (PWTs, PWTd), left ventricular end-diastolic dimension (LVDd), and left ventricular end-systolic dimension (LVDs) were measured in 2-dimensional M-mode. Pulsed wave doppler was applied to check velocities in the 4-chamber apical view. Volume sampling was positioned at the tip of the mitral valve to measure early LV filling velocity (E) and left atrial contraction velocity (A). Tissue doppler was then conducted with the volume sampling repositioning at the septal annulus of the mitral valve to measure early (E’) and late (A’) diastolic mitral annular velocities.
LV systolic function was defined by EF. The EF was calculated by the modified Simpson’s method, subtracting LVDs from LVDd. LV mass (LVM) was estimated using the Devereux modified ASE cube formula [17], and the LV mass index (LVMI) was calculated by dividing LVM by body surface area.
E/E’ and E/A were calculated to represent diastolic function. DT, the time between peak E wave and the upper deceleration slope extrapolated to zero line, was also determined. In 23 patients exhibiting atrial fibrillation (n = 21, 3.1% in non-CIN group and n = 2, 3.1% in CIN group), average measurements from 10 cardiac cycles were used [11].
Left atrial volume index (LAVI), a marker of volume status, was estimated with the biplane Simpson’s method using the diameters of the LA, which were measured 3 times at the parasternal long axis view (anterior-posterior, superior-inferior) and the 4 chamber view (medio-lateral).
Inter-reader reliability, intra-reader reliability, and reader drift analyses were performed on a random sample of 3% of the entire cohort. The intra-class correlation coefficients for the echocardiographic measures were 0.845 for EF, 0.765 for LVMI, and 0.753 for LAVI.
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) for Windows, version 18.0 (Chicago, IL, USA). Data are expressed as a mean ± standard deviation for continuous variables, and as a number and percentage for categorical variables. Normality of distribution was examined by the Shapiro-Wilk test.
We compared the demographic, laboratory, and echocardiographic parameters of patients with CIN with those of patients without CIN using the Student’s t-test, Mann–Whitney U test, or chi-square test. Either ANOVA or the Kruskal-Wallis test was performed to compare the 3 groups, which were classified according to the E/E’ values of 8 and 15. Univariate and multivariate binary logistic regression analyses were conducted to identify risk factors predicting the development of CIN. We also performed receiver operating characteristic (ROC) analysis to confirm the predictive accuracy of echocardiographic parameters for CIN. All-cause mortality was compared between patients using 4 grou**s that were based on the presence or absence of diastolic dysfunction (E/E’ > 15) and CIN, by Kaplan-Meier and log-rank test. A p-value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics between patients with CIN and those without CIN
Baseline characteristics of the study subjects are detailed in Table 1. The mean age was 64.8 ± 10.6 years and 69.9% were male. The mean values of eGFR and E/E’ were 74.5 ± 20.5 mL/min/1.73m2 and 13.3 ± 5.3, respectively. A total of 64 patients (8.7%) developed CIN.
When patients were dichotomized into CIN and non-CIN groups, the CIN group was older and had lower body mass index (BMI) than the non-CIN group. The prevalence of hypertension, DM, emergency/ urgent procedure, three-vessel coronary artery disease (3-VD), previous coronary artery bypass graft surgery, and IABP use during the PTCA was significantly higher in the CIN group compared to the non-CIN group. Within the laboratory findings, patients with CIN demonstrated eGFR, hemoglobin, and albumin levels that were significantly lower, while hs-CRP levels were significantly higher compared to the non-CIN group. Echocardiographic findings revealed that the EF and DT were decreased, while E/E’ (18.2 ± 7.0 vs. 12.9 ± 4.9, p < 0.001), LVMI, LVDd, and LAVI were significantly increased in patients with CIN.
When patients were classified into three groups based on the E/E’ values of 8 and 15, CIN occurred in 42 (21.6%) patients in the highest tertile compared with 20 (4.0%) in the middle and 2 (4.3%) in the lowest tertile (p < 0.001). In addition, patients in the highest tertile were older and had more hypertension, DM, and 3-VD. They also experienced more hypotensive events and had IABPs placed more frequently during procedure than patients in the middle and lowest tertiles. Among the echocardiographic parameters, LVMI, LVDd, and LAVI were significantly increased in the highest tertile (Table 2).
Risk factors for the development of CIN
Logistic regression analysis showed that higher E/E’ level [odds ratio (OR) 1.147, 95% confidence interval (CI) 1.101-1.194, p < 0.001 as a continuous variable/ OR 6.519, 95% CI 3.774-11.260, p < 0.001 as a categorical variable] was a significant risk factor for CIN (Table 3). After adjustment for age, BMI, hypertension, DM, emergency/ urgent procedure, 3-VD, volume of CM per weight, use of IABP, eGFR < 60 mL/min/1.73m2, hemoglobin, albumin, and hs-CRP, E/E’ still remained as an independent risk factor (OR 1.091, 95% CI 1.026-1.159, p = 0.005 as a continuous variable/ OR 3.435, 95% CI 1.522-7.755, p = 0.003 as a categorical variable). In successive models, further adjustments were made with other echocardiographic parameters, EF and LAVI. E/E’ > 15 was found to be a final determinant of CIN (OR 2.579, 95% CI 1.082-5.964, p = 0.035) (Table 4).
Defining CIN as an increase in serum creatinine ≥ 0.3 mg/dL from the baseline value, according to the AKIN criteria, did not change the independent role of E/E’ for the prediction of CIN (OR 2.456, 95% CI 1.046-6.217, p = 0.044) (see Additional file 1).
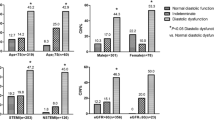
In subgroup analysis, CIN occurred most often in patients in the highest tertile of E/E’ irrespective of diabetes status (n = 15, 13.6% in non-DM group, p < 0.001/ n = 27, 32.1% in DM group, p < 0.001). A similar finding was observed in patients with an eGFR < 60 mL/min/1.73m2 (n = 35, 47.3% in the highest tertile of E/E’, p < 0.001), although there was no difference in the development of CIN among the three tertiles of E/E’ in patients with an eGFR ≥ 60 mL/min/1.73m2 (p = 0.199) (Figure 1).
Receiver operating characteristic analysis of E/E’ for the development of CIN
To estimate the predictive accuracy of the echocardiographic parameters for the development of CIN, ROC analysis was performed. The area under the curve (AUC) for E/E’, EF, and LAVI were 0.75 (95% CI 0.68-0.82, p < 0.001), 0.74 (95% CI 0.67-0.81, p < 0.001), and 0.66 (95% CI 0.58-0.74, p < 0.001), respectively (Figure 2).
When AKIN criteria were applied, estimated AUC of E/E’ for CIN was 0.79 (95% CI 0.73-0.85, p < 0.001) (see Additional file 2).
Survival analysis in CIN and diastolic dysfunction
During a mean follow-up of 17.9 months, 30 patients died. The most common causes of death were cardiovascular disease (n = 16, 53.5%), followed by infection (n = 12, 40%).
Kaplan-Meier analysis showed higher mortality rates in patients who developed CIN (n = 18, 28.1% vs. n = 11, 1.6%). In addition, there was a tendency toward higher mortality in those with diastolic dysfunction and CIN (n = 14, 33.3%) (Figure 3). The two-year survival rates were 98.1% in patients without CIN or diastolic dysfunction, 95.8% in patients with diastolic dysfunction but no CIN, 72.6% in subjects with CIN and normal diastolic function, and 58.1% in patients with both CIN and diastolic dysfunction (p < 0.001).
Comparison of E/E’ with previous risk scoring systems for CIN
We reclassified our patients according to the risk stratification systems suggested by Mehran et al. [14] and Bartholomew et al. [4]. The incidence of CIN was well proportional to both risk scores. Predictive performances for CIN, estimated by AUC on ROC analysis, were about 80% with previous methods and 75% with E/E’ (see Additional file 3).
Discussion
This study showed that patients with CIN exhibited a lower EF and higher E/E’ and LAVI on echocardiography. In addition, the highest tertile of E/E’ was associated with a significantly increased risk of CIN, beyond the well-known risk factors such as DM and renal failure. This implicates that diastolic dysfunction may be a useful parameter for predicting the development of CIN.
There are both patient-related and patient-unrelated factors in the risk stratification systems for CIN. Patient-related factors include chronic kidney disease (CKD), DM, emergency/ urgent procedure, IABP use, CHF, age > 75 years, hypertension, anemia, a hypotensive episode, and LVEF < 40%. Patient-unrelated factors include using ionic and hyper-osmolar contrast agent, and high volume of CM [9, 14]. These factors play a role in the development of CIN primarily by reducing effective renal blood flow, consequently causing hypoxic change and the synthesis of reactive oxygen species. Development of CIN has also been attributed to increased sympathetic tone, renin-angiotensin-aldosterone system (RAAS) activation, the overproduction of many humoral factors such as vasopressin, catecholamines, endothelin, and pro-inflammatory cytokines, and decreased nitric oxide (NO) levels. These hemodynamic and neurohumoral alterations can cause vascular endothelial cell damage and hypertrophic change in the vascular smooth muscle cells, thus further aggravating blood flow disturbances [5, 18].
Although CHF is a well-proven risk factor for CIN, only low ejection fraction has previously been studied, among various components of heart failure such as diastolic dysfunction, ventricular hypertrophy, and volume overload [7, 8, 19]. This has likely been because a decreased effective circulatory volume is considered the primary event in the development of CIN, in patients with CHF [20, 21]. However, the statistical significance of EF disappeared in the multivariate logistic regression analysis, whereas E/E’ remained a significant risk factor for CIN (see Additional file 4). ROC analysis also showed a considerable predictive accuracy for E/E’, which was comparable to that of EF (Figure 2). Moreover, most studies demonstrated that EF only affects CIN rates in patients with severe CHF of NYHA class 3–4 or an EF < 30-40%. Given that CIN occurs in higher rates in CHF patients with lower severity, this suggests that the hemodynamic compromise status in milder stages of heart failure cannot be properly represented by EF [8]. Actually, CIN occurred in 18.2% of patients with EF < 40%, and 7.8% of patients with EF ≥ 40% in our study. However, when patients with severe CHF were excluded, E/E’ still was an independent risk factor for CIN (OR 2.593, 95% CI 1.014-6.633, p = 0.041, data not shown). This finding suggests that diastolic dysfunction may be a more reliable parameter to represent the hemodynamic and neurohumoral alterations observed in CHF.
Studies conducted in patients with CHF and preserved LVEF showed a proportional increase in renal failure according to the diastolic dysfunction [22]. The stiffer the LV, the faster the eGFR declined [23]. The main reasons for this include reduced LV functional reserve, resting/ exercise-exacerbated systolic dysfunction, and chronotropic incompetence, which are responsible for the decrease in blood flow. These result in insufficient tissue perfusion and an ischaemic injury to the kidney [24–26]. Initially, LV hypertrophy occurs to compensate for the stiffness of the LV, by increasing stroke volume. But pathologic proliferation, fibrotic deposition, and calcification of the ventricle become evident, and LV compliance eventually decreases [27]. The same vicious cycle in hemodynamics is thought to be the chief mechanism for a higher E/E’ causing CIN. The adverse effect of diastolic dysfunction on patient mortality is partly attributed to these unfavorable geometric changes in the LV (Figure 3).
On the other hand, the neurohumoral changes occurring during the development of CIN further aggravate diastolic dysfunction. Angiotension II and aldosterone promote the growth/ proliferation of both cardiomyocytes and non-myocyte cells like fibroblasts [28]. Pro-inflammatory cytokines such as TNF-α and IL-6 stimulate collagen production by fibroblasts, and bring about a myocardio-depressive effect [29]. We also demonstrated elevated hs-CRP levels in patients in the highest tertile of E/E’ (Table 2). In addition, increased sympathetic tone activates the β-catenin pathway in the cardiomyocytes through the recruitment of Akt (protein kinase B). This up-regulates the production of osteoblastogenic proteins, further aggravating diastolic function [30]. Through these mutual effects on hemodynamic and neurohumoral status, higher E/E’ is thought to cause acute kidney injury when patients with CHF are exposed to the CM.
Furthermore, it should be noted that older age and increased prevalences of hypertension, DM, and 3-VD were present in subjects with diastolic dysfunction (Table 2). These comorbidities are well-proven risk factors, and may potentiate the relationship between diastolic dysfunction and CIN. In DM, advanced glycation endproduct (AGE) mediates the crosslinking of collagen fibres in the myocardium [31]. It also causes an inflammation in the renal vascular system after binding to the receptor of AGE (RAGE), through activation of NADPH oxidase, MAP kinase, and the NF-κB pathway [32, 33]. Because insulin and c-peptide are known to evoke the overexpression of inducible endothelial NO synthase, impaired pancreatic secretion is also expected to increase E/E’ and exacerbate renal hemodynamics [34, 35]. Actually, several studies demonstrated an improved ejection fraction/ diastolic function after correcting for these metabolic abnormalities in diabetic CKD patients with a kidney-pancreas co-transplantation [36, 37]. Underlying hypertension and CKD also strengthen the linkage between diastolic dysfunction and CIN through the effects of uremic toxin [38, 39], pressure overload, RAAS activation, and markedly elevated expression of humoral mediators such as catecholamines, endothelin, and parathyroid hormone [40].
There are several shortcomings to our study. Because this was a retrospective study, limited data were available, which may have affected the conclusion by type II error. Second, as estimating LVEDP during the PTCA was not a routine practice in our institute, LVEDP and their relationships with E/E’ and CIN could not be presented. However, in other small cohort of 55 patients who measured LVEDP during the PTCA in our hospital, E/E’ levels were well correlated with LVEDP (r = 0.78, p < 0.001). The ROC analysis demonstrated that the predictive accuracy of E/E’ for LVEDP > 15mmHg was 0.88 (p < 0.001, 95% CI 0.794-0.981). E/E’ > 15 had 81% sensitivity and 86% specificity for LVEDP > 15 mmHg (see Additional file 5). These results correspond closely with previous studies by Sohn et al. and Ommen et al. [11–13]. Moreover, E/E’ can be measured non-invasively prior to PTCA, and warns the physicians to estimate LVEDP during procedure and to monitor patients’ renal function carefully after procedure, in advance, when levels are elevated. Third, as only patients who were suspected to have CAOD and had undergone an echocardiography were included, selection bias may have existed. Higher incidence rate of CIN in our study group compared to that in previous studies conducted in the general population supports this [1]. However, considering that interventional procedures using intravascular CM are most closely related with the development of CIN, and most patients requiring such procedures have underlying cardiac disease and DM, the results of present study can be applied to the high-risk patients planning for the angioplasty. The uncontrolled use of medications with vasomotor action or that modulate the neurohumoral axis is another limitation. However, former clinical trials using RAAS blockade, statins, calcium channel blockers, beta-blockers, and diuretics failed to derive a common consensus as to their effects on CIN [41–43]. Lastly, as it was a single-center study, further large-scale, randomized controlled multicenter trials are needed to confirm and assess the clinical applicability of our findings.
Conclusions
In conclusion, we demonstrated that, among echocardiographic parameters, E/E’ can be a useful predictor for the development of CIN. To our knowledge, this is the first study suggesting that assessment of diastolic dysfunction should play a role in the risk stratification for CIN.
Abbreviations
- AGE:
-
Advanced glycation endproduct
- AKIN:
-
Acute Kidney Injury Network
- ANP:
-
Atrial natriuretic peptide
- ASE:
-
American Society of Echocardiography
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CAOD:
-
Coronary artery occlusive disease
- CHF:
-
Congestive heart failure
- CI:
-
Confidence interval
- CIN:
-
Contrast-induced nephropathy
- CKD:
-
Chronic kidney disease
- CM:
-
Contrast media
- DT:
-
Deceleration time
- EF:
-
Ejection fraction
- eGFR:
-
Estimated glomerular filtration rate
- Hs-CRP:
-
High-sensitivity C-reactive protein
- IABP:
-
Intra-aortic balloon pump
- IVS:
-
Interventricular septal thickness
- LAVI:
-
Left atrial volume index
- LVDd:
-
Left ventricular end-diastolic dimension
- LVDs:
-
Left ventricular end-systolic dimension
- LVEDP:
-
Left ventricular end-diastolic pressure
- LVM:
-
Left ventricular mass
- LVMI:
-
Left ventricular mass index
- MDRD:
-
Modification of Diet in Renal Disease
- NO:
-
Nitric oxide
- NYHA:
-
New York Heart Association
- OR:
-
Odds ratio
- PTCA:
-
Percutaneous transluminal coronary angioplasty
- PWT:
-
Posterior wall thickness
- RAAS:
-
Renin-angiotensin-aldosterone system
- ROC:
-
Receiver operating characteristic
- 3-VD:
-
Three-vessel coronary artery disease.
References
Goldfarb S, McCullough PA, McDermott J, Gay SB: Contrast-induced acute kidney injury: specialty-specific protocols for interventional radiology, diagnostic computed tomography radiology, and interventional cardiology. Mayo Clin Proc Mayo Clin. 2009, 84: 170-179.
Finn WF: The clinical and renal consequences of contrast-induced nephropathy. Nephrol, Dial, Transplantat. 2006, 21: i2-10.
McCullough PA: Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008, 51: 1419-1428.
Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O'Neill WW: Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004, 93: 1515-1519.
Goldenberg I, Matetzky S: CMAJ: Can Med Assoc J = Journal de l'Association Medicale Canadienne. 2005, 172: 1461-1471.
Mehran R, Nikolsky E: Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. 2006, 100: 11-15.
Rosenstock JL, Gilles E, Geller AB, Panagopoulos G, Mathew S, Malieckal D, DeVita MV, Michelis MF: Impact of heart failure on the incidence of contrast-induced nephropathy in patients with chronic kidney disease. Int Urol Nephrol. 2010, 42: 1049-1054.
Nikolsky E, Mehran R, Turcot D, Aymong ED, Mintz GS, Lasic Z, Lansky AJ, Tsounias E, Moses JW, Stone GW, et al: Impact of chronic kidney disease on prognosis of patients with diabetes mellitus treated with percutaneous coronary intervention. Am J Cardiol. 2004, 94: 300-305.
Pannu N, Wiebe N, Tonelli M: Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006, 295: 2765-2779.
Barrett BJ, Parfrey PS: Clinical practice. Preventing nephropathy induced by contrast medium. New Engl J Med. 2006, 354: 379-386.
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A: Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009, 10: 165-193.
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ: Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000, 102: 1788-1794.
Sohn DW, Song JM, Zo JH, Chai IH, Kim HS, Chun HG, Kim HC: Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. J Am Soc Echocardiogr. 1999, 12: 927-931.
Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, et al: A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004, 44: 1393-1399.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007, 11: R31-
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999, 130: 461-470.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N: Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986, 57: 450-458.
Tumlin J, Stacul F, Adam A, Becker CR, Davidson C, Lameire N, McCullough PA: Pathophysiology of contrast-induced nephropathy. Am J Cardiol. 2006, 98: 14K-20K.
Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, et al: Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005, 95: 13-19.
Heyman SN, Rosenberger C, Rosen S: Regional alterations in renal haemodynamics and oxygenation: a role in contrast medium-induced nephropathy. Nephrol, Dial, Transplant. 2005, 20 (Suppl 1): i6-11.
Gami AS, Garovic VD: Contrast nephropathy after coronary angiography. Mayo Clin Proc Mayo Clin. 2004, 79: 211-219.
Victor BM, Barron JT: Diastolic heart failure versus diastolic dysfunction: difference in renal function. Clin Cardiol. 2010, 33: 770-774.
de Sa DD C, Hodge DO, Slusser JP, Redfield MM, Simari RD, Burnett JC, Chen HH: Progression of preclinical diastolic dysfunction to the development of symptoms. Heart. 2010, 96: 528-532.
Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM: Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010, 56: 845-854.
Borlaug BA, Paulus WJ: Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011, 32: 670-679.
Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, et al: Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009, 54: 402-409.
Afshinnia F, Spitalewitz S, Chou SY, Gunsburg DZ, Chadow HL: Left ventricular geometry and renal function in hypertensive patients with diastolic heart failure. Am J Fidney Dis. 2007, 49: 227-236.
Gajarsa JJ, Kloner RA: Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011, 16: 13-21.
Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL: Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010, 56: 225-231.
Fang D, Hawke D, Zheng Y, **a Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z: Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007, 282: 11221-11229.
Hartog JW, Voors AA, Bakker SJ, Smit AJ, Van Veldhuisen DJ: Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. Eur J Heart Fail. 2007, 9: 1146-1155.
Basta G, Schmidt AM, De Caterina R: Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004, 63: 582-592.
Meerwaldt R, Links T, Zeebregts C, Tio R, Hillebrands JL, Smit A: The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovasc Diabetol. 2008, 7: 29-
Kamikawa A, Ishii T, Shimada K, Makondo K, Inanami O, Sakane N, Yoshida T, Saito M, Kimura K: Proinsulin C-peptide abrogates type-1 diabetes-induced increase of renal endothelial nitric oxide synthase in rats. Diabetes Metab Res Rev. 2008, 24: 331-338.
Nordquist L, Brown R, Fasching A, Persson P, Palm F: Proinsulin C-peptide reduces diabetes-induced glomerular hyperfiltration via efferent arteriole dilation and inhibition of tubular sodium reabsorption. Am J Physiol Renal Physiol. 2009, 297: F1265-1272.
Fiorina P, La Rocca E, Astorri E, Lucignani G, Rossetti C, Fazio F, Giudici D, Di Carlo V, Cristallo M, Pozza G, Secchi A: Reversal of left ventricular diastolic dysfunction after kidney-pancreas transplantation in type 1 diabetic uremic patients. Diabetes Care. 2000, 23: 1804-1810.
Perseghin G, Fiorina P, De Cobelli F, Scifo P, Esposito A, Canu T, Danna M, Gremizzi C, Secchi A, Luzi L, Del Maschio A: Cross-sectional assessment of the effect of kidney and kidney-pancreas transplantation on resting left ventricular energy metabolism in type 1 diabetic-uremic patients: a phosphorous-31 magnetic resonance spectroscopy study. J Am Coll Cardiol. 2005, 46: 1085-1092.
Stewart GA, Gansevoort RT, Mark PB, Rooney E, McDonagh TA, Dargie HJ, Stuart R, Rodger C, Jardine AG: Electrocardiographic abnormalities and uremic cardiomyopathy. Kidney Int. 2005, 67: 217-226.
Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H: Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes?. Eur Heart J. 2010, 31: 1771-1779.
Dickhout JG, Carlisle RE, Austin RC: Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ Res. 2011, 108: 629-642.
Zhang L, Lu Y, Wu B, Zhang S, Jiang H, Ge J, Chen H: Efficacy of statin pretreatment for the prevention of contrast-induced nephropathy: a meta-analysis of randomised controlled trials. Int J Clin Pract. 2011, 65: 624-630.
Gleeson TG, Bulugahapitiya S: Contrast-induced nephropathy. AJR Am J Roentgenol. 2004, 183: 1673-1689.
Kiski D, Stepper W, Brand E, Breithardt G, Reinecke H: Impact of renin-angiotensin-aldosterone blockade by angiotensin-converting enzyme inhibitors or AT-1 blockers on frequency of contrast medium-induced nephropathy: a post-hoc analysis from the Dialysis-versus-Diuresis (DVD) trial. Nephrol, Dial, Transplant. 2010, 25: 759-764.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2369/14/146/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HMK designed the study, acquired the data, performed the statistical analysis, interpreted the analytical results, and wrote the manuscript; SHH, THY and SWK edited and made critical revision to the manuscript; HJO assisted in the study design and helped draft the manuscript; BSK participated in the data analysis and interpretation; FMD, KIK, CHK, and MJL assisted in the study design and contributed to the data acquisition; KHC organized the data collection and edited the manuscript. All authors approved the final version of the manuscript.
Electronic supplementary material
12882_2012_577_MOESM1_ESM.docx
Additional file 1: Univariate logistic regression analysis for contrast-induced nephropathy, which was defined according to the AKIN criteria.(DOCX 23 KB)
12882_2012_577_MOESM2_ESM.tiff
Additional file 2: Receiver operating characteristic curves for CIN, which was defined according to the AKIN criteria. The AUCs of EF, E/E, and LAVI were 0.70, 0.79, and 0.69, respectively (p < 0.001). (TIFF 1 MB)
12882_2012_577_MOESM3_ESM.tiff
Additional file 3: Predictive performances for CIN using various risk scoring systems. The incidence of CIN was well proportional to both (a) Mehran’s (renal failure was scored according to the eGFR: white bar, or the Cr levels: black bar) and (b) Bartholomew’s risk scores. (c) E/E’ showed a considerable predictive power for the development of CIN, which was comparable to other risk stratification methods. The AUCs of E/E’ , Mehran’s score, and Bartholomew’s score were 0.75, 0.80, and 0.82, respectively (p < 0.001). (TIFF 2 MB)
12882_2012_577_MOESM4_ESM.docx
Additional file 4: Multivariate logistic regression analysis for contrast-induced nephropathy (detailed descriptions of Table 4). (DOCX 29 KB)
12882_2012_577_MOESM5_ESM.tiff
Additional file 5: Correlation between E/E’ and LVEDP. (a) E/E’ showed a significant positive relationship with LVEDP on correlation analysis. (b) Across increasing E/E’ tertiles, LVEDP levels were incrementally higher. (c) ROC analysis revealed that the predictive accuracy of E/E’ for LVEDP > 15 mmHg was 0.88 (p < 0.001, 95% CI 0.794-0.981). (TIFF 307 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Koo, H.M., Doh, F.M., Ko, K.I. et al. Diastolic dysfunction is associated with an increased risk of contrast-induced nephropathy: a retrospective cohort study. BMC Nephrol 14, 146 (2013). https://doi.org/10.1186/1471-2369-14-146
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2369-14-146