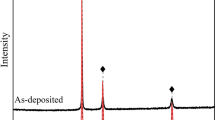
Abstract
Aircraft and aero-engine applications have manipulated nickel aluminide coatings for their corrosion resistance and oxidation resistance in high-temperature thermal barrier coatings. The purpose of this investigation is to develop a novel diffusion barrier pure Al-rich β-NiAl bond coat. To coat the K-403 superalloy substrate, both with and without a pre-electro-deposited nickel layer, aluminide coatings are deposited using in situ chemical vapor deposition (CVD). The material characterization techniques, scanning electron microscopy, energy-dispersive spectroscopy and X-ray diffraction patterns have revealed double-layered structure, outer-layer Al-rich β-NiAl and inner inter-diffusion zone. The outer-layer β-NiAl coatings formed onto the K-403 superalloy substrate is hyper-stoichiometric aluminum rich 44.76 at% Al without nickel coating. It was found that the inter-diffusion zone (IDZ) had a thickness of about 8 µm. The alloying element concentration increases steadily from exterior to the interior of the coating’s depth and develops impure Al-rich β-NiAl coatings. On the contrary, the outer-layer β-NiAl coatings formed onto the K-403 superalloy substrate are hyper-stoichiometric aluminum-rich 44.92 at% Al with nickel coating. It was found that the inter-diffusion zone (IDZ) had a thickness of about 3 µm. The Al-rich β-NiAl phase formed at the surface of the aluminized substrate is pure because nickel-deposited layer constrained the depletion of substrate.










Similar content being viewed by others
Data availability
The data that support the findings of this study are publicly available, and the correspondence author can provide upon request. Authors are agreed to process the manuscript for review.
References
J.H. Perepezko, The hotter the engine, the better. Science 326(5956), 1068–1069 (2009)
W.W. Bathie, Fundamentals of gas turbines, 2nd edn. (Wiley, 1996)
F.S. Pettit, C.S. Giggins, Hot corrosion, in Superalloys II: high-temperature materials for aerospace and industrial power. ed. by C.T. Sims, N.S. Stoloff, W.C. Hagel (Wiley, 1987)
D.R. Clarke, C.G. Levi, Materials design for the next generation thermal barrier coatings. Annu. Rev. Mater. Res. 33, 383–417 (2003)
D.R. Clarke, M. Oechsner, N.P. Padture, Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 37(10), 891–902 (2012)
J.T. Demasimarcin, D.K. Gupta, Protective coatings in the gas-turbine engine. Surf. Coat. Technol. 68–69, 1–9 (1994)
R. Vassen, M.O. Jarligo, T. Steinke, D.E. Mack, D. Stover, Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 205(4), 938–942 (2010)
W. Zhu, L. Yang, J.W. Guo, Y.C. Zhou, C. Lu, Numerical study on interaction of surface cracking and interfacial delamination in thermal barrier coatings under tension. Appl. Surf. Sci. 315, 292–298 (2014)
W.G. Mao, J. Wan, C.Y. Dai, J. Ding, Y. Zhang, Y.C. Zhou, C. Lu, Evaluation of microhardness, fracture toughness and residual stress in a thermal barrier coating system: a modified vickers indentation technique. Surf. Coat. Technol. 206, 4455–4461 (2007)
K. Loeffel, L. Anand, A chemo-thermo-mechanically coupled theory for elastic-viscoplastic deformation, diffusion, and volumetric swelling due to a chemical reaction. Int. J. Plast 27, 1409–1431 (2011)
L. Yang, Z.C. Zhong, J. You, Q.M. Zhang, Y.C. Zhou, W.Z. Tang, Acoustic emission evaluation of fracture characteristics in thermal barrier coatings under bending. Surf. Coat. Technol. 232, 710–718 (2013)
I.A. Abro, M.I. Abro, M.E. Assad, M. Rahimi-Gorji, N.M. Hoang, Investigation and evaluation of neem leaves extract as a green inhibitor for corrosion behavior of mild steel: An experimental study, Proceedings of the Institution of Mechanical Engineers, part C, 235, 734–743, (2021)
D. Salehi Doolabi, M.R. Rahimipour, M. Alizadeh, S. Pouladi, S.M.M. Hadavi, M.R. Vaezi, Effect of high vacuum heat treatment on microstructure and cyclic oxidation resistance of HVOF-CoNiCrAlY coatings. Vacuum 135, 22–33 (2017)
R. Sitek, K. Sikorski, J.W. Sobczak, T. Wierzchoń, Structure and properties of the multilayers produced on Inconel 600 by PACVD method with the participation of trimethylaluminium vapors. Mater. Sci. Poland 26(3), 767–777 (2008)
A.S. Ulrich, M.C. Galetz, Protective aluminide coatings for refractory metals. Oxid. Met. 86, 511–535 (2016)
Z. Xu, Z. Wang, J. Niu, J. Dai, L. He, R. Mu, Phase structure, morphology evolution and protective behaviors of chemical vapor deposited (Ni, Pt)Al coatings. J. Alloys Compounds 676, 231–238 (2016)
R. Sitek, T. Bolek, R. Dobosz, T. Plocinski, J. Mizera, Microstructure and oxidation resistance of aluminide layer produced on Inconel 100 nickel alloy by CVD method. Surface Coat. Technol. 304, 584–591 (2016)
D. Li, H. Gou, D. Wang, T. Zhang, S. Gong, H. Xu, Cyclic oxidation of β-NiAl with various reactive element dopants at 1200°C. Corros. Sci. 66, 125–135 (2013)
R. Sitek, H. Matysiak, J. Ferenc, K.J. Kurzydłowski, Structure and properties of nickel aluminide layers on INCONEL 100. Mater. Sci. Forum 636–637, 1011–1018 (2010)
J. Klower, U. Brill, U. Hueber, High temperature corrosion behavior of nickel aluminides: effects of chromium and zirconium. Intermetallics 7, 1183–1194 (1999)
G.R. Krishna, D.K. Das, V. Singh, S.V. Joshi, Role of Pt content in the microstructural development and oxidation performance of Pt-aluminide coatings produced using a high-activity aluminizing process. Mater. Sci. Eng., A 251, 40–47 (1998)
J. Angenete, K. Stiller, A comparative study of two inward grown Pt modified Al diffusion coatings on a single crystal Ni base superalloy. Mater. Sci. Eng., A 316, 182–194 (2001)
Y. Zhang, J.A. Haynes, W.Y. Lee, I.G. Wright, B.A. Pint, K.M. Cooley, P.K. Liaw, Synthesis and cyclic oxidation behavior of a (Ni, Pt) Al coating on a desulfurized Ni-base superalloy. Metall. and Mater. Trans. A. 30, 2679–2687 (1999)
B.M. Warnes, Reactive element modified chemical vapor deposition low activity platinum aluminide coatings. Surf. Coat. Technol. 146–147, 7–12 (2001)
M.N. Task, B. Gleeson, F.S. Pettit, G.H. Meier, Compositional effects on the type I hot corrosion of β-NiAl alloys. Surf. Coat. Technol. 206, 1552–1557 (2011)
S. Kim, Y.A. Chang, An inter-diffusion study of a NiAl alloy using single-phase diffusion couples. Metall. and Mater. Trans. A. 31, 1519–1524 (2000)
H. Wei, X. Sun, Q. Zhenk, H. Guan, Z. Hu, Estimation of inter-diffusivity of the NiAl phase in Ni–Al binary system. Acta Mater. 52, 2645–2651 (2004)
H. Mehrer, Diffusion in intermetallics. Mater. Trans., JIM 37(6), 1259–1280 (1996)
M.M.P. Janssen, G.D. Rieck, Reaction diffusion and kirkendall-effect in the nickel-aluminum system. Trans. Metall. Soc. AIME 239(9), 1372–1385 (1967)
S. Rashidi, J.P. Choi, J.W. Stevenson, A. Pandey, R.K. Gupta, High Temperature oxidation behavior of aluminized haynes 230. Corros. Sci. 174, 108835 (2020)
B. Gregoire, G. Bonnet, F. Pedraza, Development of a new slurry coating design for the surface protection of gas turbine components. Surf. Coat. Technol. 374, 521–530 (2019)
R.J. Christensen, D.M. Lipkin, D.R. Clarke, K. Murphy, Nondestructive evaluation of the oxidation stresses through thermal barrier coatings using Cr3+ piezo-spectroscopy. Appl. Phys. Lett. 69, 3754 (1996)
J. Romanowska, M. Zagula-Yavorska, J. Sieniawski, Zirconium influence on microstructure of the aluminide coatings deposited on the nickel substrate by the CVD method. Bull. Mater. Sci. 36(6), 1043–1048 (2013)
M. Yavorska, J. Sieniawski, M. Zielińska, Functional properties of aluminide layer deposited on Inconel 713LC Ni-based super alloy in the CVD process. Arch. Metall. Mater. 56, 187–192 (2011)
M. Zielińska, J. Sieniawski, M. Yavorska, M. Motyka, Influence of chemical composition of nickel based super alloy on the formation of aluminide coatings. Arch. Metall. Mater. 56, 193–197 (2011)
G. Goward, Bone DH, Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 3, 475–495 (1971)
Z.D. **ang, J.S. Burnel-Gray, P.K. Datta, aluminide coating formation on nickel-base superalloys by pack cementation process. J. Mater. Sci. 36, 5673–5682 (2001)
ASTM-D2485–91, Standard test methods for evaluating coatings for high temperature service, volume: 06.01, (2013), DOI: https://doi.org/10.1520/D2485.
A. Zakeri, M.R. Masoumi Balashadehi, A. Sabour Rouh Aghdam, Development of hybrid electrodeposition/slurry diffusion aluminide coatings on Ni-based superalloy with enhanced hot corrosion resistance. J. Compos. Compounds 2, 1–8 (2021)
A. Thevand, S. Poize, J.P. Crousier, R. Streiff, Aluminization of nickel – Formation of intermetallic phases and Ni2Al3 coatings. J. Mater. Sci. 16, 2467–2479 (1981)
D.K. Das, V. Singh, S.V. Joshi, Evolution of aluminide coating microstructure on nickel-base cast superalloy CM-247 in a single-step high-activity aluminizing process. Metall. and Mater. Trans. A. 29, 2173–2188 (1998)
J.C. Liu, C. Wang, L.J. Tong Sotware, P.Y. Zhang, Q.L. Li, Study on the effect mechanism of aluminizing on fatigue performance of K403 nickel-based superalloy. J. Alloys Compounds 835, 155277 (2020)
F.H. Latief, K. Kakehi, H. Murakami, K. Kasai, Influence of crystallographic orientation on creep behavior of aluminide Ni-base single crystal superalloys, Proceedings of the International Symposium on Superalloys, 311–320, (2012).
H. Murakami, T. Sakai, Anisotropy of secondary reaction zone formation in aluminized Ni-based single-crystal superalloys. Scripta Mater. 59, 428–431 (2008)
T.H. Orem, Influence of crystallographic orientation on the corrosion rate of aluminum in acids and alkalies. J. Res. Natl. Bur. Stand. 58, 157 (1957)
X. Montero, A. Ishida, T.M. Meißner, H. Murakami, M.C. Galetz, Effect of surface treatment and crystal orientation on hot corrosion of a Ni-based single-crystal superalloy. Corros. Sci. 166, 108472 (2020)
Y. Yanqiu, W. Zhixun, Z. Yanchao, W. Jiapo, L. Zhenwei, Y. Zhufeng, Effect of crystallographic orientation on the corrosion resistance of Ni-based single crystal superalloys. Corros. Sci. 170, 108643 (2020)
P. Nash, M.F. Singleton, J.L. Murray, Phase Diagrams of Binary Nickel Alloys, Nash, P., Ed, ASM International: Materials Park, OH, USA, 3–11, (1991).
Y.B. Zhou, H.J. Zhang, Preparation and oxidation of an Al2O3-modified aluminide coating. Vacuum 86(9), 1353–1357 (2012)
V. Genova, G. Pedrizzetti, L. Paglia, F. Marra, C. Bartuli, G. Pulci, Diffusion aluminide coating modified via electroless nickel plating for Ni-based superalloy protection. Surface Coat. Technol. 439, 128452 (2022)
Acknowledgements
Irfan Ali Abro (PhD Scholar) is highly thankful and grateful to NED University of Engineering and Technology, Karachi, Pakistan, for generous support and facilities of this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors proclaim no conflict of interest concerning the publication of this original article. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this original article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abro, I.A., Chandio, A.D. Analysis and evolution on diffusional stability of nickel aluminide bond coat via nickel electro-plating. Eur. Phys. J. Plus 138, 229 (2023). https://doi.org/10.1140/epjp/s13360-023-03816-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-023-03816-6