Abstract
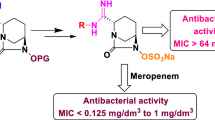
The diazabicyclooctane (DBO) has emerged as the attractive motif for the development of new β-lactamase inhibitors after the clinical approval of avibactam and relebactam. We are striving at improving the lactamase inhibition strength and antibacterial spectrum of the DBO derivatives. We synthesized the substituted-amidine derivatives (5a–5j) of the DBO, and determined the antibacterial efficacy of the two β-lactam drugs i.e. imipenem and meropenem, in combination with compounds 5a–5j. All these derivatives lowered the MIC values of the imipenem and meropenem, indicating their β-lactamase inhibition profile, however in variable strength. The best results were obtained when the compounds 5a–5j were combined with meropenem indicating the better association of these compounds with meropenem in comparison to imipenem. With meropenem, compounds 5a and 5b proved to be the most potent exhibiting the highest antibacterial efficacy against five out of ten microbial strains. Moreover, the MIC data showed that β-lactamase inhibition strength of compounds 5a and 5b was comparable to relebactam in eight out of ten bacterial strains.


Similar content being viewed by others
REFERENCES
Deja, E.N., Clin. Microbiol. Newslett., 2021, vol. 43, no. 14, p. 119. https://doi.org/10.1016/j.clinmicnews.2021.06.004
Gray, D.A. and Wenzel, M., ACS Infect. Dis., 2020, vol. 6, p. 1346. https://doi.org/10.1021/acsinfecdis.0c00001
Papp-Wallace, K.M., Mack, A.R., Taracila, M.A., and Bonomo, R.A., Infect. Dis. Clin. N. Am., 2020, vol. 34, no. 4, p. 773. https://doi.org/10.1016/j.idc.2020.05.001
Shirley, M., Drugs, 2018, vol. 78, no. 6, p. 675. https://doi.org/10.1007/s40265-018-0902-x
Coleman, K., Curr. Opin. Microbiol., 2011, vol. 14, p. 550. https://doi.org/10.1016/j.mib.2011.07.026
Falagas, M.E., Mavroudis, A.D., and Vardakas, K.Z., Expert Rev. Anti Infect. Ther., 2016, vol. 14, no. 8, p. 747. https://doi.org/10.1080/14787210.2016.1204911
Butler, M.S., and Paterson, D.L., J. Antibiot., 2020, vol. 73, p. 329. https://doi.org/10.1038/s41429-020-0291-8
Ortiz de la Rosa, J.M., Nordmann, P., and Poirel, L., J. Antimicrob. Chemother., 2019, vol. 74, no. 7, p. 1934. https://doi.org/10.1093/jac/dkz149
Chalhoub, H., Sáenz, Y., Nichols, W.W., Tulkens, P.M., and Van Bambeke, F., Int. J. Antimicrob. Agents, 2018, vol. 52, no. 5, p. 697. https://doi.org/10.1016/j.ijantimicag.2018.07.027
Davies, D.T., Leiris, S., Zalacain, M., Sprynski, N., Castandet, J., Bousquet, J., Lozano, C., Llanos, A., Alibaud, L., Vasa, S., Pattipati, R., Valige, R., Kummari, B., Pothukanuri, S., De Piano, C., Morrissey, I., Holden, K., Warn, P., Marcoccia, F., Benvenuti, M., Pozzi, C., Tassone, G., Mangani, S., Docquier, J.-D., Pallin, D., Elliot, R., Lemonnier, M., and Everett, M., J. Med. Chem., 2020, vol. 63, no. 24, p. 15802. https://doi.org/10.1021/acs.jmedchem.0c01535
Bouchet, F., Atze, H., Fonvielle, M., Edoo, Z., Arthur, M., Ethève-Quelquejeu, M., and Iannazzo, L., J. Med. Chem., 2020, vol. 63, no. 10, p. 5257. https://doi.org/10.1021/acs.jmedchem.9b02125
Gordon, E.M., Duncton, M.A.J., and Gallop, M.A., J. Med. Chem., 2018, vol. 61, no. 22, p. 10340. https://doi.org/10.1021/acs.jmedchem.8b01389
Fujiu, M., Yokoo, K., Sato, J., Shibuya, S., Komano, K., Kusano, H., Sato, S., Aoki, T., Kohira, N., Kanazawa, S., Watari, R., Kawachi, T., Hirakawa, Y., Nagamatsu, D., Kashiwagi, E., Maki, H., and Yamawaki, K., J. Med. Chem., 2021, vol. 64, no. 13, p. 9496. https://doi.org/10.1021/acs.jmedchem.1c00799
Durand-Réville, T.F., Comita-Prevoir, J., Zhang, J., Wu, X., May-Dracka, T.L., Romero, J.A.C., Wu, F., Chen, A., Shapiro, A.B., Carter, N.M., McLeod, S.M., Giacobbe, R.A., Verheijen, J.C., Lahiri, S.D., Sacco, M.D., Chen, Y., O’Donnell, J.P., Miller, A.A., Mueller, J.P., and Tommasi, R.A., J. Med. Chem. , 2020, vol. 63, no. 21, p. 12511. https://doi.org/10.1021/acs.jmedchem.0c00579
Peilleron, L., and Cariou, K., Org. Biomol. Chem., 2020, vol. 18, no. 5, p. 830. https://doi.org/10.1039/c9ob02605c
Bouchet, F., Atze, H., Arthur, M., Ethève-Quelquejeu, M., and Iannazzo, L., Org. Lett., 2021, vol. 23, no. 20, p. 7755. https://doi.org/10.1021/acs.orglett.1c02741
Gao, Y., Liu, Y., Iqbal, Z., Sun, J., Ji, J., Zhai, L., Tang, D., Ji, J., He, L., Mu, Y., Yang, H., and Yang, Z., ChemistrySelect 2021, vol. 6, no.5, p. 1174. https://doi.org/10.1002/slct.202004620
Iqbal, Z., Zhai, L., Gao, Y., Tang, D., Ma, X., Ji, J., Sun, J., Ji, J., Liu, Y., Jiang, R., Mu, Y., He, L., Yang, H., and Yang, Z., Beilstein J. Org. Chem., 2021, vol. 17, p. 711. https://doi.org/10.3762/bjoc.17.60
Sun, J., He, L., Gao, Y., Zhai, L., Ji, J., Liu, Y., Ji, J., Ma, X., Mu, Y., Tang, D., Yang, H., Iqbal, Z., and Yang, Z., Mendeleev Commun., 2021, vol. 31, no, 4, p. 498. https://doi.org/10.1016/j.mencom.2021.07.020
Liu, Y., Ji, J., Sun, J., He, L., Gao, Y., Zhai, L., Ji, J., Ma, X., Mu, Y., Tang, D., Yang, H., Iqbal, Z., and Yang, Z., Monatsh. Chem., 2022, vol. 153, p. 301. https://doi.org/10.1007/s00706-021-02888-3
Ji, J., Zhai, L., Sun, J., He, L., Ji, J., Ma, X., Liu, Y., Tang, D., Mu, Y., Gao, Y., Yang, H., Iqbal, Z., and Yang, Z., J. Heterocyclic Chem., 2021, vol. 58, no. 12, p. 2390. https://doi.org/10.1002/jhet.4360
Zhai, L., Sun, J., Ji, J., He, L., Gao, Y., Ji, J., Liu, Y., Mu, Y., Ma, X., Tang, D., Yang, H., Iqbal, Z., and Yang, Z., Russ. J. Bioorg. Chem., 2022, vol. 48, p. 1059. https://doi.org/10.1134/S1068162022050120
Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard-8th Edition. CLSI document M07-A8, Clinical and Laboratory Standards Institute Wayne, Pa., USA, 2009, vol. 29.
Ball, M., Boyd, A., Ensor, G.J., Evans, M., Golden, M., Linke, S.R., Milne, D., Murphy, R., Telford, A., Kalyan, Y., Lawton, G.R., Racha, S., Ronsheim, M., and Zhou, S.H., Org. Process Res. Dev., 2016, vol. 20, no.10, p. 1799. https://doi.org/10.1021/acs.oprd.6b00268
Kim, J., Itoh, T., Xu, F., Dance, Z.E.X., Waldman, J.H., Wallace, D.J., Wu, F., Kats-Kagan, R., Ekkati, A.R., Brunskill, A.P.J., Peng, F., Fier, P.S., Obligacion, J.V., Sherry, B.D., Liu, Z., Emerson, K.M., Fine, A.J., Jenks, A.V., and Armenante, M.E., Org. Process Res. Dev., 2021, vol. 25, no. 10, p. 2249. https://doi.org/10.1021/acs.oprd.1c00149
Nishida, H., Fujimori, I., Arikawa, Y., Hirase, K., Ono, K., Nakai, K., Inatomi, N., Hori, Y., Matsukawa, J., Fujioka, Y., Imanishi, A., Fukui, H., and Itoh, F., Bioorg. Med. Chem., 2017, vol. 25, no. 13, p. 3447. https://doi.org/10.1016/j.bmc.2017.04.034
Papp-Wallace, K.M., Endimiani, A., Taracila, M.A., and Bonomo, R.A., Antimicrob. Agents Chemother., 2011, vol. 55, no. 11, p. 4943. https://doi.org/10.1128/AAC.00296-11
Bouza, E., J. Antimicrob. Chemother., 2021, vol. 76, no. 4, p. iv38. https://doi.org/10.1093/jac/dkab353
Funding
Ministry of Science and Technology, P.R. China is gratefully Acknowledged for the award of foreign expert program to Dr. Haikang Yang and Dr. Zafar Iqbal. This work was supported by the grant from Science and Technology Department of Ningxia, P.R. China (no. 2018BCG01001).
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Rights and permissions
About this article
Cite this article
Sun, J., He, L., Ji, J. et al. Synergistic Antibacterial Activity of Meropenem and Imipenem in Combination with Diazabicyclooctane Derivatives. Russ J Gen Chem 92, 2070–2081 (2022). https://doi.org/10.1134/S1070363222100218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222100218