Abstract
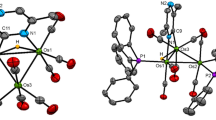
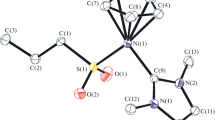
Binuclear complexes [Fe2(µ-S2ER2)(CO)6] (ER2 = SiMe2 (Iа), SiEt2 (Ib), SnEt2 (Ic)), promising precursors of heterometallic clusters, were synthesized. Reactions of these complexes with transition metal halide complexes, [Cp"RhCl2]2 (Cp" = η5-C5H3tBu2), [(Dppe)NiCl2] (Dppe = Ph2PCH2CH2PPh2), [(Ph3P)AuCl], and [Mn(CO)5Cl], were studied. The reactions gave heterometallic clusters [Fe2Rh(µ3-S)2(CO)6Cp"] (II), [Fe2Ni(µ3-S)2(CO)6(Dppe)] (III), [Fe2(CO)6(µ-SSnClEt2)(µ-SAu(PPh3))] (IV), [Fe2(CO)6(µ4,η2-S2SnEt2){Mn(CO)4Cl}2] (V), and [Fe2Mn(CO)9Mn(CO)5(µ3-S)(µ4-S)] (VI). Cluster V was found to be converted to VI upon photochemical activation. The structures of compounds I–VI were determined by X-ray diffraction (CIF file CCDC nos. 751214 (Ic), 751215 (III·0.5C7H8), 2062206 (V), 2062207 (Ib), 2062208 (Ia), 2062209 (IV·0.5CH3C5H9).



Similar content being viewed by others
REFERENCES
Schriver, D.F. and Whitmire, K.H., in Comprehensive Organometallic Chemistry, Stone, F.G.A. and Wilkinson, G, Eds., Oxford: Pergamon, 1982, p. 243.
Shieh, M. and Lai, Y.-W.J., Chin. Chem. Soc., 2002, vol. 49, p. 851.
Pasynskii, A.A. and Eremenko, I.L. Usp. Khim., 1989, vol. 58, no. 2, p. 303.
Pasynskii, A.A., Semenova, N.I., Torubaev, Yu.V., et al., Russ. Chem. Bull., 2003, vol. 52, no. 4, p. 944.
Pasynskii, A.A., Shapovalov, S.S., Tikhonova, O.A., et al., Russ. J. Coord. Chem., 2015, vol. 41, no. 11, p. 74. https://doi.org/10.1134/S1070328415110068
Torubaev, Y.V., Pasynskii, A.A., Pavlova, A.V., et al., J. Organomet. Chem., 2015, vol. 777, p. 88.
Torubaev, Y.V., Shapovalov, S.S., Tikhonova, O.G., et al., Polyhedron, 2020, vol. 177, p. 114298.
Hieber, W. and Beck, J., Z. Anorg. Allg. Chem., 1958, vol. 296, p. 91.
Seyferth, D., Henderson, R.S., and Song, L., Organometallics, 1982, vol. 1, p. 125.
Tard, C. and Pickett, C.J., Chem. Rev., 2009, vol. 109, p. 2245.
Song, L.-C., Tang, M.-Y., Mei, S.-Z., et al., Organometallics, 2007, vol. 26, p. 1575.
Song, L.-C., Wang, H.-T., Ge, J.-H., et al., Organometallics, 2008, vol. 27, p. 1409.
Nann, T., Ibrahim, S.K., Woi, P.-M., et al., Angew. Chem., Int. Ed. Engl., 2010, vol. 49, p. 1574.
Wang, F., Wang, W.-G., Wang, X.-J., et al., Angew. Chem., Int. Ed. Engl., 2011, vol. 50, p. 3193.
Wen, F., Wang, X., Huang, L., et al., ChemSusChem., 2012, vol. 5, p. 849.
Wang, F., Wang, W.-G., Wang, H.-Y., et al., ACS Catal., 2012, vol. 2, p. 407.
Simmons, T.R., Berggren, G., Bacchi, M., et al., C-oord. Chem. Rev., 2014, vols. 270−271, p. 127.
Khrizanforova, V.V., Karasik, A.A., and Budnikova, Yu.G. Russ. Chem. Bull., 2017, vol. 86, p. 298.
Hai, L., Zhang, T., Zhang, X., et al., Electrochem. Commun., 2017, vol. 82, p. 66.
Shupp, J.P., Rose, A.R., and Rose, M.J., Dalton Trans., 2017, vol. 46, p. 9163.
Arsenyeva, K.V., Ershova, I.V., Chegerev, M.G., et al., J. Organomet. Chem., 2020, vol. 927, p. 121524.
Seyferth, D., Song, L.-C., and Henderson, R.S., J. Am. Chem. Soc., 1981, vol. 103, p. 5103.
Pushkarevskii, N.A., Ogienko, M.A., Kurat’eva, N.V., and Konchenko, S.N., Russ. Chem. Bull., 2008, no. 1, p. 36.
Zhuang, B., Chen, J., He, L., et al., J. Organomet. Chem., 2003, vol. 682, p. 59.
Konchenko, S.N., Sanden, T., Pushkarevsky, N.A., et al., Chem.-Eur. J., 2010, vol. 16, p. 14278.
Dehnen, S., Eichhofer, A., and Fenske, D., Eur. J. I-norg. Chem., 2002, p. 279.
Tran, D.T.T., Kowalchuk, C.M., Taylor, N.J., and Corrigan, J.F., Inorg. Chem., 2002, vol. 41, p. 5693.
Komuro, T., Matsuo, T., Kawaguchi, H., and Tatsumi, K., Angew. Chem., 2003, vol. 115, no. 4, p. 481.
Fuhr, O., Dehnen, S., and Fenske, D., Chem. Soc. Rev., 2013, p. 1871.
Gordon, A. and Ford, R., The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References, New York: Wiley, 1972.
Busby, R., Hursthouse, M.B., Jarrett, P.S., et al., Dalton Trans., 1993, p. 3767.
Finn, M.G., Pentacarbonylchloromanganese. e-EROS Encyclopedia of Reagents for Organic Synthesis, New York: Wiley, 2001, p. 1.
Sinha, P., Wilson, A.K., and Omary, M.A., J. Am. Chem. Soc., 2005, vol. 127, p. 12488.
White, C., Yates, A., Maitlis, P.M., and Heinekey, D.M., Inorg. Synth., 1992, vol. 29, p. 228.
Seyferth, D., Henderson, R.S., Fackle, J.P., and Mazany, A.M., J. Organomet. Chem., 1981, vol. 213, p. C21.
APEX2 (version 1.08), SAINT (version 7.03), and SADABS (version 2.11), Madison: Bruker Advanced X-ray Solutions, 2004.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, no. 1, p. 112.
Groom, C.R., Bruno, I.J., Lightfoot, M.P., and Ward, S.C., Acta Crystallogr., Sect. B: Cryst. Sci., Cryst. Eng. Mater., 2016, vol. 72, p. 171.
Lozano, A.A., Santana, M.D., Garcia, G., et al., Z. Anorg. Allg. Chem., 2005, vol. 631, p. 2062.
Seyferth, D. and Gallagher, M.K., Inorg. Chim. Acta, 1983, vol. 73, p. 159.
Schmidbaur, H., Probst, T., Steigelmann, O., and Muller, G., Z. Naturforsch., A: Phys. Sci., 1989, vol. 44, p. 1175.
ACKNOWLEDGMENTS
The study was carried out within the state assignment for the Institute of Inorganic Chemistry, Siberian Branch, Russian Academy of Sciences, in the field of fundamental scientific research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of int-erest.
Additional information
Dedicated to the memory of Professor A.A. Pasynskii
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Ogienko, M.A., Pushkarevskii, N.A., Bashirov, D.A. et al. Complexes [Fe2(μ-S2ER2)(CO)6] (E = Si, Sn) as Reagents for the Synthesis of Heterometallic Clusters: Synthesis, Structure, and Reactions with Halogen-Containing Metal Complexes. Russ J Coord Chem 47, 567–577 (2021). https://doi.org/10.1134/S1070328421080042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328421080042