Abstract
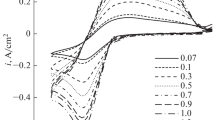
The possibility of silicon electrodeposition using LiCl–KCl–CsCl–LiF–K2SiF6 and LiCl–KCl–CsCl–K2SiF6 electrolytes is investigated. The kinetics of silicon electrowinning on glassy carbon at a temperature of 480–550°C is studied by voltammetry. Silicon electrodeposition under experimental conditions is found to proceed in one four-electron stage and to be not electrochemically reversible. The results of electrochemical measurements are used to estimate the diffusion coefficients of silicon ions in LiCl–KCl–CsCl melts (3.2 × 10–5, 7.2 × 10–6 cm2/s at temperatures of 480, 550°C, respectively) and select conditions for silicon electrodeposition. The structure and morphology of the silicon deposits formed under potentiostatic conditions are investigated. A decrease in the lithium chloride concentration in the melt is shown to result in silicon deposits in the form of dendrites and fibers.






Similar content being viewed by others
REFERENCES
A. Y. Galashev and A. S. Vorob’ev, “First principle modeling of a silicene anode for lithium ion batteries,” Electrochim. Acta 378, 138143 (2021).
Yu. P. Zaikov, S. I. Zhuk, A. V. Isakov, O. V. Grishenkova, and V. A. Isaev, “Silicon electrodeposition from the KF–KCl–KI–K2SiF6 melt,” Rasplavy, No. 5, 441–454 (2016).
M. Laptev, A. Khudorozhkova, A. Isakov, O. Grishenkova, S. Zhuk, and Yu. Zaikov, J. Serb. Chem. Soc. 86, 1–13 (2021). https://doi.org/10.2298/JSC200917065L
T. A. Gevel, S. I. Zhuk, Yu. A. Ustinova, A. V. Suzdal’tsev, and Yu. P. Zaikov, “Silicon electroreduction from the KCl–K2SiF6 melt,” Rasplavy, No. 2, 187–198 (2021).
D. B. Frolenko, Z. S. Martem’yanova, A. N. Baraboshkin, and S. V. Plaksin, “Electrodeposition of silicon from fluoride–chloride melts,” Rasplavy, No. 5, 42–49 (1993).
S. I. Zhuk, A. V. Isakov, A. P. Apisarov, O. V. Grishenkova, V. A Isaev, E. G. Vovkotrub, and Y. P. Zaykov, “Electrodeposition of continuous silicon coatings from the KF–KCl–K2SiF6 melts,” J. Electrochem. Soc. 164 (8), H5135–H5138 (2017).
A. V. Isakov, K. Chang, S. Dzhang, and Yu. P. Zaikov, “Electrochemical production of Si thin films in melts KF–KCl–KI–K2SiF6,” Tsvetn. Met., No. 11, 49–54 (2017).
O. V. Chemezov, O. N. Vinogradov-Zhabrov, A. P. Apisarov, A. V. Isakov, S. V. Plaksin, I. M. Povolotskii, A. M. Murzakaev, V. B. Malkov, and Yu. P. Zaikov, “Structure of nano and microcrystalline silicon deposits formed by electrolytic refining of Si in the KCl–CsCl–KF–K2SiF6 melt,” Perspekt. Mater.: Funkts. Nanomater. Vysokochist. Veshchestva, No. 9, 277–282 (2010).
O. V. Chemezov, A. V. Isakov, A. P. Apisarov, M. S. Brezhestovskii, O. V. Bushkova, N. N. Batalov, Yu. P. Zaikov, and A. P. Shashkin, “Electrolytic production of silicon nanofibers from the KCl–KF–K2SiF6–SiO2 melt for the composite anodes of lithium-ion batteries,” Elektrokhim. Energ. 13 (4), 201–204 (2013).
M. V. Laptev, A. V. Isakov, O. V. Grishenkova, A. S. Vorob’ev, A. O. Khudorozhkova, L. A. Akashev, and Yu. P. Zaikov, “Electrodeposition of thin silicon films from the KF–KCl–KI–K2SiF6 melt,” J. Electrochem. Soc. 167, 042506 (2020).
K. Maeda, K. Yasuda, T. Nohira, R. Hagiwara, and T. Homma, “Silicon electrodeposition in water-soluble KF–KCl molten salt: investigations on the reduction of Si(IV) ions,” J. Electrochem. Soc. 162 (9), D444–D448 (2015).
I. G. Sharma and T. K. Mukherjee, “A study on purification of metallurgical grade silicon by molten salt electrorefining,” Metall. Trans. B 17, 395–397 (1986).
J. Li, H. Ren, X. Yin, J. Lu, and J. Li, “Electrochemical behavior of Si(IV) on the Mo electrode in the CaCl2–CaF2–CaO–SiO2 melt,” Russ. J. Electrochem. 55 (5), 392–400 (2019).
S. V. Kuznetsova, V. S. Dolmatov, and S. A. Kuznetsov, “Voltammetric study of electroreduction of silicon complexes in a chloride–fluoride melt,” Russ. J. Electrochem. 45, 742–748 (2009).
G. M. Haaberg, L. Faniyeh, A. M. Martinez, and K. S. Osen, “Electrodeposition of silicon from fluoride melts,” Electrochim. Acta. 100, 226–228 (2013).
J. De Lepinay, J. Bouteillon, S. Traore, D. Renaud, and M. J. Barbier, “Electroplanting silicon and titanium in molten fluoride media,” J. Appl. Electrochem. 17, 294–302 (1987).
A. L. Bieber, L. Massot, M. Gibularo, L. Cassayre, P. Taxil, and P. Chamelot, “Silicon electrodeposition in molten fluorides,” Electrochim. Acta. 62, 282–289 (2012).
Z. Cai, Y. Li, X. He, and J. Liang, “Electrochemical behavior of silicon in the NaCl–KCl–NaF–SiO2,” Metall. Mater. Trans. B 41 (8), 1033–1137 (2010).
Z. Cai, Yu. Li, and W. Tian, “Electrochemical behavior of silicon compound in LiF–NaF–KF–Na2SiF6 molten salt,” Ionics 17, 821–826 (2011).
O. I. Boiko, Yu. K. Delimarskii, and R. V. Chernov, “Electroreduction of Si(IV) from a fluoride–chloride melt,” Ukr. Khim. Zh. 51 (4), 385–390 (1985).
V. I. Posypaiko and E. A. Alekseeva, Melting Diagrams of Salt Systems. Triple Systems (Khimiya, Moscow, 1977).
Y. Jiarong, W. Wei, and X. Wei, “Electrochemical synthesis of ammonia in molten salts,” J. Energy Chem. 43, 195–207 (2020).
E. A. Kharina, R. Yu. Kaychenkova, A. S. Dedyukhin, A. V. Shchetinskiy, and L. F. Yamshchikov, “Potentiometric study of lanthanum containing melts based on the eutectic mixture of lithium, potassium and cesium chlorides,” AIP Conf. Proc. 020037-1–020037-5 (2015).
L. Kui, S. Jiajia, Z. Ligi, and S. Yuxue, “The application of low-melting LiCl–KCl–CsCl eutectic to electrodeposit uranium metal,” J. Electrochem. Soc. 166 (13), 606–616 (2019).
V. Yu. Shishkin and V. S. Mityaev, “Purification of alkali metal halides by zone melting,” Izv. Akad. Nauk SSSR, Neorg. Mater. No. 11, 1917–1918 (1982).
A. S. Vorob’ev, A. V. Suzdal’tsev, and A. E. Galashev, “Binding energies in the molten M–Al–Zr–O–F systems (M = Li, Na, K),” Russ. Metall. (Metally), No. 8, 781–786 (2019).
A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 2001).
L. N. Antipin and S. F. Vazhenin, Electrochemistry of Molten Salts (Metallurgiya, Moscow, 1964).
ACKNOWLEDGMENTS
Spectral analysis and differential scanning calorimetry of electrolyte samples were performed using the equipment and techniques of the Matter Composition Center for Collective Use at the Institute of High-Temperature Electrochemistry, Ural Branch of the Russian Academy of Sciences.
Funding
The work was performed under agreement no. 075-03-2020-582/1 of February 18, 2020 (project no. 0836-2020-0037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by T. Gapontseva
Rights and permissions
About this article
Cite this article
Pavlenko, O.B., Ustinova, Y.A., Zhuk, S.I. et al. Silicon Electrodeposition from Low-Melting LiCl–KCl–CsCl Melts. Russ. Metall. 2022, 818–824 (2022). https://doi.org/10.1134/S0036029522080109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522080109