Abstract
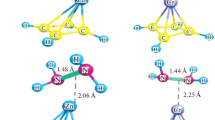
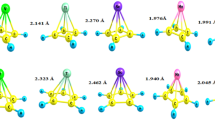
Platinum and the metals of similar row in pure or composite form are widely used as catalysts in the reaction of fuel cells. This work explored the catalytic efficiency of some organometallic compounds with the general formula TM-CmHm (TM = Cr, Sc, Ti, V and m = 4, 5) based on density function theory in hydrazine-oxygen fuel cells. PW91 method and base set 6-31G(d) were used to study the catalytic effect of the compounds mentioned in the activation of O2 on the cathode and N2H4 on the anode of the fuel cell. The results shown that O2 and N2H4 were activated in contact with the organometallic compounds to participate in the half fuel reaction. Bond length at O=O and N=N increased in response to the partial transfer of negative charge from organometallic compounds to their orbitals. The transition state calculations confirmed the feasibility of the reaction kinetically and thermodynamically.





Similar content being viewed by others
REFERENCES
A. Arsalis, Renewable Sustainable Energy Rev. 105, 391 (2019).
A. J. Slate, K. A. Whitehead, D. A. Brownson, et al., Renewable Sustainable Energy Rev. 101, 60 (2019).
A. Arshad, H. M. Ali, A. Habib, et al., Therm. Sci. Eng. Prog. 9, 308 (2019).
S. O. Ganiyu, C. A. Martínez-Huitle, and M. A. Rodrigo, Appl. Catal. B 270, 118857 (2020).
L. **ng, W. Shi, H. Su, et al., Energy 177, 445 (2019).
A. Piacentino, N. Duic, N. Markovska, et al., Energy 182, 254 (2019).
T. Lehtola and A. Zahedi, Sustain. Energy Technol. Assess 35, 25 (2019).
Y. Shao, J. P. Dodelet, G. Wu, and P. Zelenay, Adv. Mater. 31, 1807615 (2019).
A. M. Abdalla, S. Hossain, A. T. Azad, et al., Renewable Sustainable Energy Rev. 82, 353 (2018).
E. Antolini, Appl. Catal. B 237, 491 (2018).
V. C. Anitha, R. Zazpe, M. Krbal, et al., J. Catal. 365, 86 (2018).
B. Zhu, D. **a, and R. Zou, Coord. Chem. Rev. 376, 430 (2018).
V. Bon, Curr. Opin. Green Sustain. Chem. 4, 44 (2017).
X. Liu and L. Dai, Nat. Rev. Mater. 1, 1 (2016).
J. C. Mah, A. Muchtar, M. R. Somalu, and M. J. Ghazali, Int. J. Hydrogen Energy 42, 9219 (2017).
W. B. Jensen, Rubber. Chem. Technol. 55, 881 (1982).
J. L. Dutton and P. J. Ragogna, Coord. Chem. Rev. 255, 1414 (2011).
M. A. Beswick, J. S. Palmer, and D. S. Wright, Chem. Soc. Rev. 27, 225 (1982).
E. Kirillov, J. Y. Saillard, and J. F. Carpentier, Coord. Chem. Rev. 249, 1221 (2005).
W. Kaminsky, A. Funck, and H. Hähnsen, Dalton Trans. 41, 8803 (2019).
C. Janiak, Coord. Chem. Rev. 250, 66 (2006).
M. Konkol and J. Okuda, Coord. Chem. Rev. 252, 1577 (2008).
H. G. Alt, E. H. Licht, A. I. Licht, and K. J. Schneider, Coord. Chem. Rev. 250, 2 (2006).
D. Mihailovic, in Molecular Magnets Recent Highlights (Springer, Vienna, 2003), p. 21.
V. C. Gibson and S. K. Spitzmesser, Chem. Rev. 103, 283 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ahmadmahmodi, Z., Noei, M., Aghaie, M. et al. The Novel Catalytic Effect of TM-CmHm (TM = Cr, SC, Ti, V and m = 4, 5) Activation of Oxygen at the Cathode and N2H4 at the Anode in the Fuel Cell N2H4 –O2: A DFT Study. Russ. J. Phys. Chem. 96, 3025–3030 (2022). https://doi.org/10.1134/S0036024422130295
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422130295