Abstract
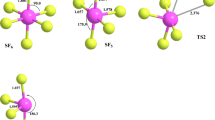
The thermal decomposition reaction of ethyl fluoroformate was investigated at two levels of theory of MP2 and DFT in the gas phase at three different proposed mechanisms. The geometry of transition state (TS) for these three mechanisms was obtained using the STQN theory and the scan method. The kinetic parameters, such as activation energy (Ea), Arrhenius rate constant (k(T)), and Arrhenius pre-exponential factor (A), were calculated for three paths of thermal decomposition based on activated complex theory. The geometry of TS was predicted for three proposed mechanisms. The results revealed that the barrier through TS1 to TS2 increased about 4–5 kJ/mol, but that through TS2 to TS3 increased between 15 and 50 kJ/mol, based on the level of theory for calculation. It means that the formation of TS3 is more difficult than the others. The calculated synchronicity values indicate that the proposed mechanisms do not correspond to a concerted and highly synchronous process.



Similar content being viewed by others
REFERENCES
D. Nouri Shargh, F. Mostafaei, Sh. Rafatpanah, and Gh. Hosseinpour, in Proceedings of the 3rd National Conference on Chemical Applications in New Technologies, Isfahan (2013), COI Code: CAAT03_167.
V. Lopez, J. Quijano, and S. Luna, Struct. Chem. 24, 1811 (2013). https://doi.org/10.1007/s11224-013-0234-0
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, et al., Gaussian 09, Revision A.1 (Gaussian Inc., Wallingford, CT, 2009).
W. Kohn, A. D. Becke, and R. G. Parr, J. Phys. Chem. 100, 12974 (1996).
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, and M. J. Frisch, J. Phys. Chem. 98, 11623 (1994).
C. Moller and M. Plesset, Phys. Rev. 46, 618 (1934).
C. Peng and H. B. Schlegel, Israel J. Chem. 33, 449 (1993).
A. E. Reed and F. Weinhold, J. Chem. Phys. 78, 4066 (1983).
A. E. Reed, L. A. Curtiss, and F. Weinhold, Chem. Rev. 88, 899 (1988).
K. B. Wiberg, Tetrahedron 24, 1083 (1968).
A. Moyano, M. A. Pericas, and E. Valenti, J. Org. Chem. 54, 573 (1989).
E. D. Glendening, A. E. Reed, J. E. Carpenter, and F. Weinhold, NBO Version 3.1 (Madison, 1988).
K. J. Glasstone, K. J. Laodler, and H. Eyring, The Theory of Rate Processes (McGraw-Hill, New York, 1941), Chap. 4.
S. W. Boston, The Foundations of Chemical Kinetics (McGraw-Hill, New York, 1969).
J. D. McQuarrie and D. A. Simon, Molecular Thermodynamics (Sausalito, CA, 1999).
F. A. Carey and R. J. Sundberg, in Advanced Organic Chemistry, Part A: Structure and Mechanisms, 5th ed. (Springer, Berlin, 2007), p. 119.
K. B. Wiberg, Tetrahedron 24, 1083 (1968).
ACKNOWLEDGMENTS
The authors would like to acknowledge the College of Science, Arak Branch, Islamic Azad University, for their support and contribution to this study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rezaie, S., Shabani, M. A Study of Factors Affecting Various Reactions of Thermal Decomposition of Ethyl Fluoroformate Using Ab Initio Quantum Mechanics Calculations. Russ. J. Phys. Chem. 95 (Suppl 2), S307–S313 (2021). https://doi.org/10.1134/S003602442115022X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442115022X