Abstract
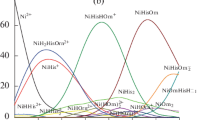
Calorimetry, spectrophotometry, and pH measurements are used to study the formation of mixed ligand complexes with 1,10-phenanthroline (Phen) and ethylenediamine (En) in Ni(II)–His–Phen(En) systems. The formation of mixed complexes with different stoichiometries is found, and the thermodynamic parameters (\(\log K\), ΔrG0, ΔrH, ΔrS) of the reactions of their formation at 298.15 K and ionic strength I = 0.5 (KNO3) are calculated. The most likely form of the coordination of the amino acid residue in the composition of the mixed complexes is identified.







Similar content being viewed by others
REFERENCES
D. S. Sigman, A. Mazumder, and D. M. Perrin, Chem. Rev. 93, 2295 (1993). https://doi.org/10.1021/cr00022a011
P. P. Silva, W. Guerra, J. N. Silveira, et al., Inorg. Chem. 50, 6414 (2011). https://doi.org/10.1021/ic101791r
P. P. Silva, W. Guerra, G. Coelho dos Santos, et al., J. Inorg. Biochem. 132, 67 (2014). https://doi.org/10.1016/j.**orgbio.2013.09.014
B. E. Fischer and H. Sigel, J. Am. Chem. Soc. 102, 2998 (1980). https://doi.org/10.1021/ja00529a021
G. S. Malik, S. P. Singh, and J. P. Tandon, Monatsh. Chem. 110, 149 (1979). https://doi.org/10.1007/BF00903758
K. Kumar, D. R. Prasad, and P. C. Nigam, Monatsh. Chem. 115, 731 (1984). https://doi.org/10.1007/BF01120968
V. K. Patel and P. K. Bhattacharya, Inorg. Chim. Acta 92, 199 (1984). https://doi.org/10.1016/S0020-1693(00)87759-2
V. A. Borodin, V. P. Vasil’ev, and E. V. Kozlovskii, Mathematic Problems of Chemical Thermodynamics (Nauka, Novosibirsk, 1985), p. 219 [in Russian].
L. D. Pettit, Pure Appl. Chem. 56, 247 (1984). https://doi.org/10.1351/pac198456020247
P. Paoletti, Pure Appl. Chem. 56, 491 (1984). https://doi.org/10.1351/pac198456040491
G. Anderegg, Helvet. Chim. Acta 46, 2813 (1963). https://doi.org/10.1002/hlca.19630460740
O. Yamauchi and A. Odani, J. Am. Chem. Soc. 107, 5938 (1985). https://doi.org/10.1021/ja00307a019
O. Yamauchi, T. Sakurai, and A. Nakahara, J. Am. Chem. Soc. 101, 4164 (1979). https://doi.org/10.1021/ja00509a024
O. Yamauchi and A. Odani, Inorg. Chim. Acta 100, 165 (1985). https://doi.org/10.1016/S0020-1693(00)88304-8
O. Yamauchi and A. Odani, J. Chem. Soc., Dalton Trans., p. 3411 (2002). https://doi.org/10.1039/B202385G
H. Masuda, T. Sugimori, O. Yamauchi, et al., Inorg. Chim. Acta 180, 73 (1991). https://doi.org/10.1016/S0020-1693(00)83068-6
D. Inci and R. Aydın, J. Sol. Chem. 50, 128 (2021). https://doi.org/10.1007/s10953-020-01046-3
E. V. Kozlovskii, D. F. Pyreu, and T. B. Khochenkova, Russ. J. Inorg. Chem. 53, 1158 (2008). https://doi.org/10.1134/S0036023608070309
D. Pyreu and S. Gridchin, J. Therm. Anal. Calorim. 139, 1435 (2020). https://doi.org/10.1007/s10973-019-08453-9
E. V. Kozlovskii, G. V. Chistyakova, and V. P. Vasil’ev, Zh. Neorg. Khim. 34, 853 (1989).
Funding
This work was supported by the RF Ministry of Science and Higher Education as part of a State Task for Ivanovo State University, notice no. FZZM-2021-0002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Pyreu, D.F., Nikitina, M.G. Thermodynamics of the Formation of Mixed Complexes of Nickel(II) Ions with Histidine and Diamines in an Aqueous Solution. Russ. J. Phys. Chem. 95, 2238–2245 (2021). https://doi.org/10.1134/S0036024421110169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421110169