Abstract
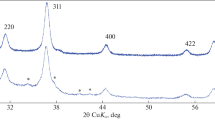
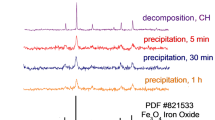
Magnetic iron oxides have been widely used in bio- and agrotechnology, however, procedures of their synthesis can be improved. Magnetic nanopowders whose composition corresponds to magnetite–maghemite solid solutions have been obtained by chemical precipitation from aqueous solutions of iron(II, III) chlorides. Synthesis conditions (exposure to ultrasound, inert gas bubbling, heating to 70°C, kee** in mother liquor) have been shown by scanning and transmission electron microscopy and by computing unit cell parameters for magnetite and maghemite lattice to affect the size, shape, and aggregation extent of nanoparticles. No monophase magnetite and maghemite formed during the synthesis, a solid solution of mixed composition has precipitated.




Similar content being viewed by others
REFERENCES
M. V. Knurova, I. Y. Mittova, N. S. Perov, et al., Russ. J. Inorg. Chem. 62, 281 (2017). https://doi.org/10.1134/S0036023617030081
A. E. Dosovitskii, E. V. Grishechkina, A. L. Mikhlin, et al., Russ. J. Inorg. Chem. 62, 702 (2017). https://doi.org/10.1134/S0036023617060055
L. V. Kozhitov, D. G. Muratov, V. G. Kostishin, et al., Russ. J. Inorg. Chem. 62, 1499 (2017). https://doi.org/10.1134/S0036023617110110
V. N. Nikiforov, A. E. Goldt, E. A. Gudilin, et al., Bull. Russ. Acad. Sci.: Phys. 78, 1075 (2014). https://doi.org/10.3103/S1062873814100141
M. V. Kulikova and V. I. Kochubei, Izv. Samarskogo Nauchn. Tsentra RAN 14, 206 (2012).
M. V. Volostnykh, A. G. Muradova, and E. V. Yurtov, Uspekhi v Khimii i Khim. Tekhnologii 25 (8), 7 (2011).
A. G. Muradova, M. P. Zaytseva, A. I. Sharapaev, and E. V. Yurtov, Colloids Surf., A 509, 229 (2016). https://doi.org/10.1016/j.colsurfa.2016.08.080
R. M. Cornell and U. Schwertmann, Book Review: The Iron Oxides von Rochelle, Acta Hydrochim. Hydrobiol. 31, (2003). https://doi.org/10.1002/aheh.200390056
A. S. Lyadov, A. A. Kochubeev, L. D. Koleva, et al., Russ. J. Inorg. Chem. 61, 1387 (2016). https://doi.org/10.1134/S0036023616110127
S. Laurent, D. Forge, M. Port, et al., Chem. Rev. 108, 2064 (2008). https://doi.org/10.1021/cr068445e
C. Xu and S. Sun, Adv. Drug. Deliv. Rev. 65, 732 (2013). https://doi.org/10.1016/j.addr.2012.10.008
M. Rui, C. Ma, Y. Hao, et al., Front. Plant Sci. 7, 815 (2016). https://doi.org/10.3389/fpls.2016.00815
N. G. M. Palmqvist, G. A. Seisenbaeva, P. Svedlindh, et al., Nanoscale Res. Lett. 12, 631 (2017). https://doi.org/10.1186/s11671-017-2404-2
G. G. Panova, O. A. Shilova, A. M. Nikolaev, et al., Agrofizika No. 3, 40 (2019). https://doi.org/10.25695/AGRPH.2019.03.07
E. Darezereshki, Mater. Lett. 64, 1471 (2010).
R. Rotaru, P. Samoila, N. Lupu, et al., Rev. Roum. Chim. 62, 131 (2017).
H. Rashid, M. A. Mansoor, B. Haider, et al., Sep. Sci. Technol. (Philadelphia, PA, U. S.) 54, (2019). https://doi.org/10.1080/01496395.2019.1585876
S. Liu, G. Wu, H. Chen, and M. Wang, Synth. Met. 162, 89 (2012).
A. Cervellino, R. Frison, G. Cernuto, et al., J. Appl. Crystallogr. 47, 1755 (2014). https://doi.org/10.1107/S1600576714019840
S. Nasrazadani and A. Raman, Corros. Sci. 34, 1355 (1993).
C. Pecharroman, T. Gonzalez-Carreno, and J. E. Iglesias, Phys. Chem. Miner. 22, 21 (1995).
J. W. Anthony, R. A. Bideaux, and K. W. Bladh, Magnetite. Handbook of Mineralogy (Mineralogical Society of America, Chantilly, VA, 2018).
ACKNOWLEDGMENTS
The results of transmission electron microscopy were obtained using equipment of the Shared Facility Center, Voronezh State University. http://ckp.vsu.ru
Funding
This work was supported by the Russian Science Foundation (project no. 19-13-00442
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Shilova, O.A., Nikolaev, A.M., Kovalenko, A.S. et al. Synthesis of Magnetic Nanopowders of Iron Oxide: Magnetite and Maghemite. Russ. J. Inorg. Chem. 65, 426–430 (2020). https://doi.org/10.1134/S0036023620030134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620030134