Abstract
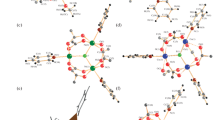
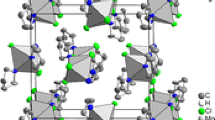
Thulium trifluoroacetate compounds have been synthesized, Tm(CF3COO)3 · 3H2O (I) and Tm2(CF3COO)6 · 2CF3COOH · 3H2O (II). The structure of I has been refined by the Rietveld method on the basis of the structural data for Cd(CF3COO)3 · 3H2O. The structure of II has been solved in a single-crystal X-ray diffraction study. Compound I has been studied by thermal analysis. Crystals of I and II are monoclinic: for I a = 9.062(2) Å, b = 18.678(3) Å, c = 9.687(2) Å, β = 113.93(1)°, Z = 2, space group P21/c, R 1 = 0.062; for II a = 8.560(4) Å, b = 19.866(5) Å, c = 20.813(7) Å, β = 101.69(4)°, Z = 8, space group C2/c, R 1 = 0.0392. In the molecular structure of I, thulium atoms are bonded in pairs through four bridging trifluoroacetate anions to form dimers. The coordination polyhedron of the thulium atom also includes the three O atoms of the water molecules and the O atom of the monodentate trifluoroacetate group; the coordination number of the thulium atom is eight. In the chain structure of II, there are two crystallographically independent thulium atoms with coordination numbers 8 and 9. The coordination polyhedra of the Tm(1) and Tm(2) atoms are a distorted monocapped tetragonal antiprism and a distorted tetragonal antiprism, respectively. The Tm-O bond lengths are in the range 2.28(1)–2.85(2) Å. The thulium atoms are bound into chains through carboxylate groups. These chains are linked into layers through hydrogen bonds.
Similar content being viewed by others
References
M. A. Porai-Koshits, Itogi Nauki Tekh., Ser. Kristallokhim. 15, 3 (1981).
S. Fujihara, M. Tada, and T. Kimura, J. Sol-Gel Sci. Technol. 19, 311 (2000).
B. Barja, R. Baggio, M. T. Garland, P. F. Aramendia, O. Penà, and M. Perec, Inorg. Chim. Acta, No. 346, 187 (2003).
V. Ya. Kavun, T. A. Kaidalova, V. I. Kostin, E. S. Panin, and B. N. Chernyshov, Coord. Chem. 10, 1502 (1984).
G. V. Romanenko, N. P. Sokolova, and S. V. Larionov, J. Struct. Chem. 40, 387 (1999).
P. C. Junk, C. J. Kepert, L. Wei-Min, B. W. Skelton, and A. H. White, Aust. J. Chem. 52, 459 (1999).
J. Zhang, S. Zhang, X. Miao, G. Wei, N. Hu, and Z. **, Chin. J. Chem. 5, 30 (1988).
S. R. Drake, A. Lyons, D. J. Otway, and D. J. Williams, Inorg. Chem. 33, 1230 (1994).
A. A. Rastorguev, A. A. Remova, G. V. Romanenko, N. P. Sokolova, V. I. Belyi, and S. V. Larionov, J. Struct. Chem. 42, 907 (2001).
F. Izumi, The Rietveld Method, Ed. by R. A. Young (Oxford Univ. Press, New York, 1993), Chapter 13.
G. M. Sheldrick, SHELXS97 and SHELXL97 (Univ. of Göttingen, Göttingen, 1997).
M. Singh, S. N. Misra, and R. D. Verma, J. Am. Chem. Soc. 40, 1939 (1978).
J. E. Roberts, J. Am. Chem. Soc. 83, 1087 (1961).
K. F. Belyaeva, M. A. Porai-Koshits, T. I. Malinovskii, L. A. Aslanov, L. S. Sukhanova, and L. I. Martynenko, Zh. Strukt. Khim., No. 10, 557 (1969).
G. G. Sadikov, G. A. Kukina, and M. A. Porai-Koshits, Zh. Strukt. Khim., No. 8, 551 (1967).
P. C. Junk and J. C. Kepert, Aust. J. Chem. 52, 459 (1999).
Author information
Authors and Affiliations
Additional information
Original Russian Text © S.I. Gutnikov, E.V. Karpova, M.A. Zakharov, A.I. Boltalin, 2006, published in Zhurnal Neorganicheskoi Khimii, 2006, Vol. 51, No. 4, pp. 593–600.
Rights and permissions
About this article
Cite this article
Gutnikov, S.I., Karpova, E.V., Zakharov, M.A. et al. Thulium(III) trifluoroacetates Tm(CF3COO)3 · 3H2O and Tm2(CF3COO)6 · 2CF3COOH · 3H2O: Synthesis and crystal structure. Russ. J. Inorg. Chem. 51, 541–548 (2006). https://doi.org/10.1134/S0036023606040061
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0036023606040061