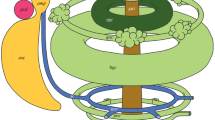
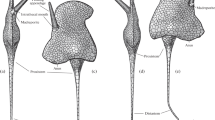
Abstract
Within all major taxa of Bilateria, there are forms with coelomic metamerism. This suggests that coelomic metamerism was characteristic of the common ancestor of Bilateria. Among deuterostomes, metamerism is clearly expressed in chordates, and elements of metamerism are present in hemichordates. Do echinoderms have remnants of coelomic metamerism that was inherited from the common ancestor of Bilateria? The coelomic system of echinoderms includes several metameric coelomic rings located along the oral-aboral axis, namely: the axocoelomic ring, the hydrocoelomic ring, 2 to 6 coelomic rings originating from the left somatocoel, and one epigastric ring originating from the right somatocoel. Thus, in echinoderms, there is a dissymmetrical metamerism, derived from the original metamerism of the common ancestors of Deuterostomia and, possibly, the common ancestors of Bilateria. The problem of dexiothetism as the cause for the formation of coelomic dissymmetry in echinoderms is discussed.





Similar content being viewed by others
REFERENCES
Adachi, S., Niimi, I., Sakai, Y., Sato, F., Minokawa, T., Urata, M., Sehara-Fujisawa, A., Kobayashi, I., and Yamaguchi, M., Anteroposterior molecular registries in ectoderm of the echinus rudiment, Dev. Dyn., 2018, vol. 247, pp. 1297–1307.
Adoutte, A., Balavoine, G., Lartillot, N., Lespinet, O., Prud’homme, B., and de Rosa, R., The new animal phylogeny: reliability and implications, Proc. Nat. Acad. Sci. U. S. A., 2000, vol. 97, no. 9, pp. 4453–4456.
Aguinaldo, A.M.A., Turbeville, J.M., Lindford, L.S., Rivera, M.C., Garey, J.R., Raff, R.A., et al., Evidence for a clade of nematodes, arthropods and other moulting animals, Nature, 1997, vol. 387, pp. 489–493.
Arendt, D., Hox genes and body segmentation. An ancient gene cluster controls the formation of repetitive body parts in a sea anemone, Science, 2018, vol. 361, no. 6409, pp. 1377–1380.
Baguñà, J., Martinez, P., Paps, J., and Riutort, M., Back in time: a new systematic proposal for the Bilateria, Philos. Trans. R. Soc., B, 2008, vol. 363, pp. 1481–1491.
Balavoine, G., Segment formation in annelids: patterns, processes and evolution, Int. J. Dev. Biol., 2014, vol. 58, pp. 469–483.
Balavoine, G. and Adoutte, A., The segmented Urbilateria: a testable scenario, Integr. Comp. Biol., 2003, no. 43, pp. 137–147.
Balser, E.J. and Ruppert, E.E., Ultrastructure of axial vascular and coelomic organs in Comasterid Featherstars (Echinodermata: Crinoidea), Acta Zool. (Stockholm), 1993, vol. 74, no. 2, pp. 87–101.
Balser, E.J., Ruppert, E.E., and Jaeckle, W.B., Ultrastructure of auricularia larval coeloms: evidence for the presence of an axocoel, Biol. Bull., 1993, vol. 185, no. 1, pp. 86–96.
Barrois, J., Recherches sur le Développement de la Comatule (C. mediterranea), Rec. Zool. Suisse, 1888, no. 4, pp. 545–651.
Bather, F.A., The Echinoderma, in A Treatise on Zoology, London: Adam and Charles Black, 1900, pt. 3.
Beaster-Jones, L., Horton, A.C., Gibson-Brown, J.J., Holland, N.D., and Holland, L., The amphioxus T-box gene, AmphiTbx15/18/22, illuminates the origins of chordate segmentation, Evol. Dev., 2006, vol. 8, no. 2, pp. 119–129.
Beklemishev, V.N., Osnovy sravnitel’noi anatomii bespozvonochnykh. Tom 1. Promorfologiya (Principles of Comparative Anatomy of Invertebrates, Vol. 1: Promorphology), Moscow: Nauka, 1964; Beklemishev, V.N., Osnovy sravnitel’noi anatomii bespozvonochnykh. Tom 2. Organologiya (Principles of Comparative Anatomy of Invertebrates, Vol. 2: Organology), Moscow: Nauka, 1964.
Beneden van, E., Recheres sur le développment des Arachnactis. Contribution à la morphologie de Cérianthides, Archs. Biol. Paris, 1891, no. 11, pp. 115–146.
Benito, J. and Pardos, F., Hemichordata, New York: Wiley, 1997, vol. 15, pp. 15–101.
Blair, S.S., Segmentation in animals, Curr. Biol., 2008, vol. 18, no. 21, pp. R991–995.
Brooks, W.K. and Grave, C., Ophiura brevispina, Mem. Nat. Acad. Sci. Wash., 1899, no. 5, pp. 79–100.
Burdon-Jones, C., Development and biology of the larva of Saccoglossus horsti (Enteropneusta), Philos. Trans. R. Soc., B, 1952, vol. 236, pp. 553–589.
Bury, H., The early stages in the development of Antedon rosacea, Philos. Trans. R. Soc., B, 1888, no. 179, pp. 257–301.
Bury, H., Metamorphosis of echinoderms, Quart. J. Microsc. Sci., 1895, no. 38, pp. 45–137.
Cameron, R.A., Rowen, L., Nesbitt, R., Bloom, S., Rast, J.P., Berney, K., Arenas-Mena, C., Martinez, P., Lucas, S., Richardson, P.M., Davidson, E.H., Peterson, K.J., and Hood, L., Unusual gene order and organization of the sea urchin Hox cluster, J. Exp. Zool. B (Mol. Dev. Evol.), 2006, vol. 306, pp. 45–58.
Chia, F.S., The embryology of a brooding starfish Leptasterias hexactis Stimpson, Acta Zool., 1968, vol. 49, no. 3, pp. 321–364.
Clark, H.L., Synapta vivipara, a contribution of the morphology of echinoderms, Mem. Boston Soc. Nat. Hist., 1898, no. 5, pp. 53–88.
Couso, J.P., Segmentation, metamerism and the Cambrian explosion, Int. J. Dev. Biol., 2009, no. 53, pp. 1305–1316.
Cuénot, L., Études anatomiques et morphologiques sur les ophiures, Arch. Zool. Exp. Gen. Ser., 1888, no. 6, pp. 33–82.
Cuénot, L., Études morphologiques sur les echinoderms, Arch. Biol., 1891, no. 11, pp. 313–680.
David, B. and Mooi, R., How Hox genes can shed light on the place of echinoderms among the deuterostomes, EvoDevo, 2014, vol. 5, p. 22.
Davis, G.K. and Patel, N.H., The origin and evolution of segmentation, Trends Cell Biol., 1999, no. 9, pp. M68–M72.
Dunn, C.W., Hejnol, A., Matus, D.Q., Pang, K., Browne, W.E., Smith, S.A., et al., Broad phylogenomic sampling improves resolution of the animal tree of life, Nature, 2008, vol. 452, no. 7188, pp. 745–749.
Dunn, C.W., Giribet, G., Edgecombe, G.D., and Hejnol, A., Animal phylogeny and its evolutionary implications, Annu. Rev. Ecol. Evol. Syst., 2014, vol. 45, no. 1, pp. 371–395.
Ezhova, O.V. and Malakhov, V.V., The nephridial hypothesis of the gill slit origin, J. Exp. Zool. B (Mol. Dev. Evol.), 2015, no. 324, pp. 647–652.
Ezhova, O.V. and Malakhov, V.V., Axial complex of Crinoidea: comparison with other Ambulacraria, J. Morphol., 2020, vol. 281, no. 11, pp. 1456–1475.
Ezhova, O.V., Lavrova, E.A., and Malakhov, V.V., Microscopic anatomy of the axial complex in the starfish Asterias rubens (Echinodermata, Asteroidea), Biol. Bull. (Moscow), 2013, vol. 40, no. 8, pp. 643–653. https://doi.org/10.1134/S1062359013080049
Ezhova, O.V., Lavrova, E.A., and Malakhov, V.V., The morphology of the axial complex and associated structures in Asterozoa (Asteroidea, Echinoidea, Ophiuroidea), Russ. J. Mar. Biol., 2014, vol. 40, no. 3, pp. 153–164. https://doi.org/10.1134/S1063074014030043
Ezhova, O.V., Lavrova, E.A., Ershova, N.A., and Mala-khov, V.V., Microscopic anatomy of the axial complex and associated structures in the brittle star Ophiura robusta Ayres, 1854 (Echinodermata, Ophiuroidea), Zoomorphology, 2015, vol. 134, no. 2, pp. 247–258.
Ezhova, O.V., Egorova, E.A., and Malakhov, V.V., Transformations of the axial complex of ophiuroids as a result of shifting of the madreporite to the oral side, Biol. Bull. (Moscow), 2016, vol. 43, no. 6, pp. 494–502. https://doi.org/10.1134/S1062359016060091
Ezhova, O.V., Ershova, N.A., and Malakhov, V.V., Microscopic anatomy of the axial complex and associated structures in the sea cucumber Chiridota laevis Fabricius, 1780 (Echinodermata, Holothuroidea), Zoomorphology, 2017, vol. 136, no. 2, pp. 205–217.
Ezhova, O.V., Malakhov, V.V., and Egorova, E.A., Axial complex and associated structures of the sea urchin Strongylocentrotus pallidus (Sars, G.O. 1871) (Echinodermata: Echinoidea), J. Morphol., 2018, vol. 279, no. 6, pp. 792–808.
Fedotov, D.M., On the problem of the homology of echinoderms, enteropneusts, and chordates, Izv. Biol. Nauchno-Issled. Inst. Perm. Univ., 1923, vol. 2, no. 1, pp. 1–11.
Fedotov, D.M., Zur morphologie des axialen organkomplexes der Echinodermen, Z. Wiss. Zool., 1924, vol. 123, pp. 209–304.
Fedotov, D.M., Tip iglokozhikh (Echinodermata). Rukovodstvo po zoologii (Phylum Echinodermata: Zoology Guide), Moscow: Sovetskaya Nauka, 1951, vol. 3, no. 2, pp. 460–591.
Franz, V., Morphologie der Akranier, Ergebn. Anat. und Entwickl. Gesch., 1927, vol. 27, pp. 464–568.
Gemmill, J.F., The development of the starfish Solaster endeca Forbes, Trans. Zool. Soc., 1912, vol. 20, no. 1, pp. 1–71.
Gemmill, J.F., The development and certain points in the adult structure of the starfish Asterias rubens L, Philos. Trans. R. Soc. London, 1914, no. 205, pp. 213–294.
Gemmill, J.F., Double hydrocoele in the development and metamorphosis in the larva of Asterias rubens L., Quart. J. Microsc. Sci., 1915, no. 61, pp. 51–80.
Gemmill, J.F., The development of the starfish Crossaster papposus Müller and Troschel, Quart. J. Microsc. Sci., 1920, no. 64, pp. 155–189.
Giribet, G., New animal phylogeny: future challenges for animal phylogeny in the age of phylogenomics, Org. Diversity Evol., 2015, vol. 16, no. 2, pp. 419–426.
Gislén, T., Affinities between the Echinodermata, Enteropneusta and Chordonia, Zool. Bijdr. Upps., 1930, vol. 12, pp. 199–304.
Goethe, J.W., Zur Naturwissenschaft uberhaupt, besonders zur Morphologie: Erfahrung, Betrachtung, Folgerung, durch Lebensereignisse verbunden, Stuttgart: J.E. Gotta, 1817, vol. 1.
Goethe, J.W., Izbrannye sochineniya po estestvoznaniyu (Selected Works on Natural Science), Moscow: Akad. Nauk SSSR, 1957.
Goto, S., The metamorphosis of Asterias pallida with special reference to the fate of the body cavities, J. Coll. Sci. Imp. Univ. Tokyo, 1898, no. 10, pp. 239–278.
Halanych, K.M., The new view of animal phylogeny, Annu. Rev. Ecol. Evol. Syst., 2004, vol. 35, pp. 229–256.
Halanych, K.M., Bacheller, J.D., Aguinaldo, A.M.A., Liva, S.M., Hillis, D.M., and Lake, J.A., Evidence from 18s ribosomal DNA that the lophophorates are protostome animals, Science, 1995, vol. 267, no. 5204, pp. 1641–1643.
Hamann, O., Beiträge zur Histologie der Echinodermen, No. 3: Die Anatomie und Histologie der Echiniden und Spatangiden, Jena: G. Fischer, 1887.
Hatschek, B., Studien über Entwicklung des Amphioxus, Arb. Zool. Inst. Univ. Wien Zool. Sta. Triest., 1881, vol. 4, pp. 1–88.
He, S., del Viso, F., Chen, C.-Y., Ikmi, A., Kroesen, A.E., and Gibson, M.C., An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis, Science, 2018, vol. 361, pp. 1377–1380.
Heinzeller, T. and Welsch, U., Microscopic Anatomy of Invertebrates, Vol. 14: Echinodermata, New York: Wiley, 1994.
Hérouard, E., Recherches sur les holothuries des côtes de France, Arch. Zool. Exp. Gen. Ser., 1889, no. 7, pp. 573–704.
Hessling, R. and Westheide, W., Are Echiura derived from a segmented ancestor? Immunohistochemical analysis of the nervous system in developmental stages of Bonellia viridis, J. Morphol., 2002, vol. 252, pp. 100–113.
Holland, L.Z., Holland, N.D., and Gilland, E., Amphioxus and the evolution of head segmentation, Integr. Comp. Biol., 2008, vol. 48, no. 5, pp. 630–646.
Horst van der, C.J. Hemichordata, in Klassen und Ordnungen des Tierreichs, Bronns, H.G., Ed., Leipzig: Leipzig. Akademische Verlagsgesellschaft M. B. H., 1939.
Hörstadius, S., Über die Entwicklung von Astropecten aurantiacus L., Pubbl. Staz. Zool. Napoli, 1939, vol. 17, no. 2, pp. 221–312.
Hyman, L.H., Echinodermata, Vol. 4 of The Invertebrates. New York: McGraw-Hill, 1955.
Hyman, L.H., Phylum Hemichordata, Vol. 5 of The Invertebrates: Smaller Coelomate Groups. New York: McGraw-Hill, 1959, pp. 72–207.
Ivanova-Kazas, O.M., Sravnitel’naya embriologiya bespozvonochnykh zhivotnykh. Tom 3. Iglokozhie i polukhordovye (Comparative Embryology of Invertebrates, Vol. 3: Echinodermata and Hemichordata), Moscow: Nauka, 1978.
Jefferies, R.P.S., A new calcichordate from the Ordovician of bohemia and its anatomy, adaptations and relationships, Biol. J. Linn. Soc., 1972, no. 4, pp. 69–115.
Jefferies, R.P.S., The Ancestry of the Vertebrates, London: Br. Mus. Nat. Hist., 1986.
Jefferies, R.P.S., A defence of the calcichordates, Lethaia, 1997, vol. 30, pp. 1–10.
Jefferies, R.P.S., Brown, N.A., and Daley, P.E.J., The early phylogeny of chordates and echinoderms and the origin of chordate left-right asymmetry and bilateral symmetry, Acta Zool. (Stockholm), 1996, vol. 77, .
Kaul-Strehlow, S. and Stach, T., A detailed description of the development of the hemichordate Saccoglossus kowalevskii using SEM, TEM, histology and 3D-reconstructions, Front. Zool., 2013, vol. 10, no. 53, pp. 1–31.
Kikuchi, M., Omori, A., Kurokawa, D., and Akasaka, K., Patterning of anteroposterior body axis displayed in the expression of Hox genes in sea cucumber Apostichopus japonicus, Dev. Gen. Evol., 2015, vol. 225, no. 5, pp. 275–286.
Kolata, D.R., Frest, T.J., and Mapes, R.H., The youngest Carpoid: occurrence, affinities, and life mode of a Pennsylvanian (Morrowan) Mitrate from Oklahoma, J. Paleontol., 1991, vol. 65, no. 5, pp. 844–855.
Kristof, A., Wollesen, T., and Wanninger, A., Segmental mode of neural patterning in Sipuncula, Curr. Biol., 2008, no. 18, pp. 1129–1132.
Lameere, A., Une théorie zoologique, Bull. Sci. Fr. Belg., 1916, no. 40, pp. 378–431.
Ludwig, H., Neue Beiträge zur Anatomie der Ophiuren, Z. Wiss. Zool., 1880, vol. 34, pp. 57–89.
Lankester, E. Ray and Willey, A., The development of the atrial chamber of Amphioxus, Quart. J. Microsc. Sci. New Ser., 1890, vol. 31, pp. 445–466.
MacBride, E.W., The development of Asterina gibbosa, Quart. J. Microsc. Sci., 1896, no. 38, pp. 339–411.
MacBride, E.W., The development of Echinus esculentus, Philos. Trans. R. Soc., 1903, no. 195, pp. 285–327.
MacBride, E.W., The development of Ophiothrix fragilis, Quart. J. Microsc. Sci., 1907, no. 51, pp. 557–606.
Malakhov, V.V., The problem of the basic body plant in various groups of deuterostomes, Zh. Obshch. Biol., 1977, vol. 38, no. 4, pp. 485–499.
Malakhov V.V. The problem of the origin of echinoderms in the light of data on their embryonic development, in Problemy izucheniya iskopaemykh i sovremennykh iglokozhikh (Problems of the Study of Fossil and Modern Echinoderms), Tallinn: Akad. Nauk Est. SSR, Inst. Geol., 1989, pp. 14–23.
Malakhov V.V. New ideas on the origin of bilateral animals, Russ. J. Mar. Biol., 2004, vol. 30, supp. 1, pp. 22–33.
Malakhov, V.V., Revolution in zoology: a new system of Bilateria, Priroda, 2009, no. 3, pp. 40–54.
Malakhov, V.V., A new system of Bilateria, Vestn. Ross. Akad. Nauk, 2010, vol. 80, no. 1, pp. 27–44.
Malakhov, V.V., Revolution in zoology: new ideas about the system and phylogeny of multicellular animals, Vestn. Ross. Akad. Nauk, 2013, vol. 83, no. 3, pp. 210–215.
Malakhov, V.V. and Cherkasova, I.V., Metamorphosis of the sea cucumber Stichopus japonicus (Aspidochirota, Stichopodidae), Zool. Zh., 1992, vol. 71, no. 9, pp. 11–21.
Mazet, F. and Shimeld, S.M., The evolution of chordate neural segmentation, Dev. Biol., 2002, vol. 251, pp. 258–270.
Minelli, A., Introduction: the evolution of segmentation, Arthropod Struct. Dev., 2017, vol. 46, no. 3, pp. 323–327.
Mooi, R. and David, B., Radial symmetry, the anterior/posterior axis, and echinoderm Hox genes, Ann. Rev. Ecol. Evol. Syst., 2008, vol. 39, pp. 43–62.
Mortensen, T., Studies in the development of crinoids, in Papers from the Department of Marine Biology, Washington: Carnegie Institution of Washington, 1920, no. 16, pp. 1–94.
Muller, W.A., Goethe als vergleichender Morphologe: Der Zwischenkiefer des Menschen und die Wirbeltheorie des Schadels, in R-Evolution—des Biologischen Weltbildes bei Goethe, Kant und ihren Zeitgenossen, Berlin: Springer-Verlag, 2015, pp. 53–67.
Narasimhamurti, N., The development of Ophiocoma nigra, Quart. J. Microsc. Sci., 1933, no. 76, pp. 63–88.
Nosenko, T., Schreiber, F., Adamska, M., Adamski, M., Eitel, M., Hammel, J., et al., Deep metazoan phylogeny: when different genes tell different stories, Mol. Phylogenet. Evol., 2013, vol. 67, no. 1, pp. 223–233.
Oken, L., Uber die Bedeutung der Schadelknochen: ein Programm beim Antritt der Professur, Jena: J.C.G. Gotfried, 1807.
Olsen, H., The development of the brittle-star Ophiopholis aculeata with a short report on the outer hyaline layer, Bergens Mus. Arb., 1942, no. 6, pp. 1–107.
Olssona, L., Ericsson, R., and Cerny, R., Vertebrate head development: segmentation, novelties, and homology, Theory Biosci., 2005, vol. 124, pp. 145–163.
Onai, T., The evolutionary origin of chordate segmentation: revisiting the enterocoel theory, Theory Biosci., 2018, vol. 137, no. 1, pp. 1–16.
Onai, T., Aramaki, T., Inomata, H., Hirai, T., and Kuratani, S., On the origin of vertebrate somites, Zool. Lett., 2015, vol. 1, p. 33. https://doi.org/10.1186/s40851-015-0033-0
Onai, T., Adachi, N., and Kuratani, S., Metamerism in cephalochordates and the problem of the vertebrate head, Int. J. Dev. Biol., 2017, vol. 61, pp. 621–632.
Osterud, H.L., Preliminary observations on the development of Leptasterias hexactis, Publ. Puget Sound Biol., 1918, no. 2, pp. 1–15.
Peel, A. and Akam, M., Evolution of segmentation: rolling back the clock, Curr. Biol., 2003, no. 13, pp. R708–R710.
Peterson, K.J. and Eernisse, D.J., Animal phylogeny and the ancestry of Bilaterians: inferences from morphology and 18s rDNA gene sequences, Evol. Dev., 2001, vol. 3, no. 3, pp. 170–205.
Philippe, H., Lartillot, N., and Brinkmann, H., Multigene analyses of Bilaterian animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa and Protostomia, Mol. Biol. Evol., 2005, vol. 22, no. 5, pp. 1246–1253.
De Robertis, E.M., The ancestry of segmentation, Nature, 1997, no. 387, pp. 25–26.
De Robertis, E.M., The molecular ancestry of segmentation mechanisms, Proc. Natl. Acad. Sci. U. S. A., 2008, vol. 105, no. 43, pp. 16411–16412.
De Rosa, R., Grenier, J.K., Andreeva, T., Cook, C.E., Adoutte, A., Akam, M., Carroll, S.B., and Balavoine, G., Hox genes in brachiopods and priapulids and protostome evolution, Nature, 1999, vol. 399, pp. 772–776.
Rozhnov, S.V., Historical development of echinoderm symmetry: from primary bilateral-asymmetric metamerism to pentamerism, in Morfogenez v individual’nom i istoricheskom razvitii: simmetriya i asimmetriya (Morphogenesis in Individual and Historical Development: Symmetry and Asymmetry), Ser. Geo-Biol. Sist. Proshlom, Moscow: Paleontol. Inst., Ross. Akad. Nauk, 2013, pp. 181–203.
Rozhnov, S.V., Morphogenesis and evolution of crinoids and other pelmatozoan echinoderms in the Early Paleozoic, Paleont. J., 2002, vol. 36, no. 6, pp. 525–674.
Rozhnov, S.V., Symmetry of echinoderms: from initial bilaterally-asymmetric metamerism to pentaradiality, Nat. Sci., 2014, vol. 6, no. 4, pp. 171–183.
Runnström, S., Über die Entwicklung von Leptosynapta inhaerens (O.Fr. Müller), Bergens Mus. Arb, 1927, no. 1, pp. 1–80.
Ruppert, E.E., Barnes, R.D., and Fox, R.S., Echinodermata, in Invertebrate Zoology, Belmont: Thomson Brooks/Cole, 2004, vol. 28, pp. 872–929.
Sedgwick, A., On the nature of metameric segmentation and some other morphological questions, Quart. J. Microsc. Sci., 1884, no. 24, pp. 43–82.
Seeliger, O., Studien zur Entwicklungsgeschichte der Crinoiden (Antedon rosacea), Zool. J. Abt. Anat. Ontog., 1892, vol. 6, pp. 161–444.
Selenka, E., Beitrage zur anatomie und systematik der holothurien, Z. Wiss. Zool., 1867, vol. 17, pp. 291–374.
Smith, A.B., Classification of the Echinodermata, Palaeontology, 1984, vol. 27, no. 3, pp. 431–459.
Smith, S.A., Wilson, N.G., Goetz, F.E., Feehery, C., Andrade, S.C.S., Rouse, G.W., Giribet, G., and Dunn, C.W., Resolving the evolutionary relationships of molluscs with phylogenomic tools, Nature, 2011, vol. 480, pp. 364–367.
Snodgrass, R.E., Evolution of the Annelida, Onychophora and Arthropoda, Smithson. Misc. Collect., 1938, no. 97, pp. 1–159.
Sprinkle, J., Morphology and evolution of blastozoan echinoderms, Harv. Univ., Mus. Comp. Zool., Spec. Publ., 1973, pp. 1–283.
Stokes, M.D. and Holland, N.D., Embryos and larvae of a lancelet, Branchiostoma floridae, from hatching through metamorphosis: growth in the laboratory and external morphology, Acta Zool., 1995, vol. 76, no. 2, pp. 105–120.
Tautz, D., Segmentation, Dev. Cell, 2004, no. 7, pp. 301–312.
Telford, M.J., Budd, G.E., and Philippe, H., Phylogenomic insights into animal evolution, Curr. Biol., 2015, vol. 25, pp. R876–R887.
Thompson, W., On the embryology of Antedon rosaceus, Philos. Trans. R. Soc. London, 1865, no. 155, pp. 513–544.
Ubaghs, G., General characters of Echinodermata, in Treatise on Invertebrate Paleontology, Part S: Echinodermata 1, Lawrence: Univ. of Kansas Press, 1967, pp. 3–60.
Ubisch, L., Die Entwicklung von Strongylocentrotus lividus (Echinus microtuberculatus, Arbacia pustulosa), Z. Wiss. Zool., 1913, vol. 106, pp. 409–448.
Wanninger, A., Twenty years into the “new animal phylogeny:” changes and challenges, Org. Diversity Evol., 2016, vol. 16, pp. 315–318.
Wanninger, A., Kristof, A., and Brinkmann, N., Sipunculans and segmentation, Commun. Integr. Biol., 2009, vol. 2, pp. 56–59.
Willey, A., The later larval development of Amphioxus, Quart. J. Microsc. Sci., 1891, vol. 32, no. 126, pp. 183–230.
Yastrebov, S.A., Vertebrate head metamerism: the current state of an old problem, Biol. Bull. (Moscow), 2018, vol. 97, no. 8, pp. 904–915.
Ziegler, A., Faber, C., and Bartolomaeus, T., Comparative morphology of the axial complex and interdependence of internal organ systems in sea urchins (Echinodermata: Echinoidea), Front. Zool., 2009, vol. 6, no. 10, pp. 1–31.
ACKNOWLEDGMENTS
We are grateful to Alexander Semenov (Pertsov Biological Station, Moscow State University) for providing photographs of echinoderms.
Funding
The preparation of the manuscript and illustrations was supported by the Russian Science Foundation (RSF, project no. 18-74-10025). Analysis of the branchial apparatus of Deuterostomia and participation of V.V. Malakhov was supported by the Russian Foundation for Basic Research, project no. 20-04-00909-a). The study was performed within the framework of the state assignment of Moscow State University (project no. 121032300121-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Ezhova, O.V., Malakhov, V.V. Coelom Metamerism in Echinodermata. Paleontol. J. 55, 1073–1083 (2021). https://doi.org/10.1134/S0031030121100038
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031030121100038