Abstract
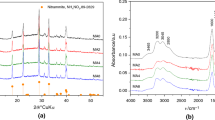
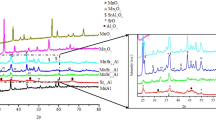
The catalytic activity of alumina-manganese catalysts in the oxidation of CO was studied. The MnO x -Al2O3 catalysts were prepared by an extrusion method with the introduction of mechanically activated components (manganese oxide and its mixtures with aluminum oxide, aluminum hydroxide, and a mixture of a manganese salt with aluminum hydroxide) into a paste of aluminum hydroxide followed by thermal treatment in air or argon at 1000°C. In the majority of cases, the catalysts contained a mixture of the phases of β-Mn3O4 (Mn2O3), α-Al2O3, and δ-Al2O3. The presence of low-temperature δ-Al2O3 suggested the incomplete interaction of manganese and aluminum oxides. It was found that the catalytic activity of MnO x -Al2O3 depends on the degree of interaction of the initial reactants, and its value is correlated with the amount of β-Mn3O4 in the active constituent. The intermediate thermal treatment of components at 700°C negatively affects the catalytic activity as a result of the formation of Mn2O3 and the coarsening of particles, which levels the results of mechanochemical activation. The greatest degree of interaction between Al- and Mn-containing components was reached in the selection of mechanochemical activation conditions by decreasing the size of grinding bodies, optimizing the time of mechanochemical activation, and using the mechanochemical activation of precursor mixtures. As a result of mechanochemical activation, the initial reactants were dispersed, the amounts of MnO2 and Mn2O3 changed, and defects were formed; this strengthened the interaction of components and increased catalytic activity.
Similar content being viewed by others
References
Tsyrul’nikov, P.G., Sal’nikov, V.A., Drozdov, V.A., Stuken, S.A., Bubnov, A.V., Grigorov, E.I., Kalinkin, A.V., and Zaikovskii, V.I., Kinet. Katal., 1991, vol. 32, p. 439.
Tsyrul’nikov, P.G., Ross. Khim. Zh., 2007, no. 4, p. 133.
RF Patent 2063803, 1994.
RF Patent 2365408, 2009.
Tsybulya, S.V., Krukova, G.N., Vlasov, A.A., Boldyreva, N.N., Kovalenko, O.N., and Tsyrulnikov, P.G., React. Kinet. Catal. Lett., 1998, vol. 64, no. 1, p. 113.
Bulavchenko, O.A., Tsybulya, S.V., Tsyrul’nikov, P.G., Afonasenko, T.N., Cherepanova, S.V., and Gerasimov, E.Yu., J. Struct. Chem., 2010, vol. 51, no. 3, p. 500.
Molchanov, V.V. and Buyanov, R.A., Russ. Chem. Rev., 2000, vol. 69, no. 5, p. 435.
Avvakumov, E.G., Khim. Interes. Ustoich. Razvit., 1994, no. 2, p. 541.
Baklanova, O.N., Bogdanets, E.N., Lavrenov, A.V., and Buluchevskii, E.A., Mekhanokhimicheskaya aktivatsiya tverdykh tel: Primenenie v sinteze geterogennykh katalizatorov (Mechanochemical Activation of Solids: Application to the Synthesis of Heterogeneous Catalysts), Omsk: Omsk. Gos. Teknol. Univ., 2012.
Shirokov, Yu.G., Mekhanokhimiya v tekhnologii katalizatorov (Mechanochemistry in Catalyst Production Technology), Ivanovo: Ivanov. Gos. Khim.-Tekhnol. Univ., 2005.
Isupova, L.A., Sadykov, V.A., Pauli, I.A., Andryushkova, O.V., Poluboyarov, V.A., Litvak, G.S., Kryukova, G.N., Burgina, U.V., Solov’eva, L.P., and Kolomiichuk, V.N., Mekhanokhimiya i mekhanokhimicheskaya aktivatsiya: Tezisy dokl. mezhd. seminara (Proc. Int. Workshop on Mechanochemistry and Mechanochemical Activation), St. Petersburg, 1995, p. 153.
Tsybulya, S.V., Kryukova, G.N., Kriger, T.A., and Tsyrul’nikov, P.G., Kinet. Catal., 2003, vol. 44, no. 2, p. 287.
Dekker, E.H.L.J. and Rieck, G.D., Z. Anorg. Allg. Chem., 1975, vol. 415, p. 69.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.A. Bulavchenko, T.N. Afonasenko, P.G. Tsyrul’nikov, O.A. Knyazheva, O.N. Baklanova, S.V. Tsybulya, 2014, published in Kinetika i Kataliz, 2014, Vol. 55, No. 5, pp. 671–680.
Rights and permissions
About this article
Cite this article
Bulavchenko, O.A., Afonasenko, T.N., Tsyrul’nikov, P.G. et al. MnO x -Al2O3 catalysts for deep oxidation prepared with the use of mechanochemical activation: The effect of synthesis conditions on the phase composition and catalytic properties. Kinet Catal 55, 639–648 (2014). https://doi.org/10.1134/S0023158414050048
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158414050048