Abstract
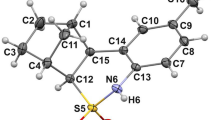
Configuration lability of the nitrogen atom in the –N(H)–SO2– group of the cyclic sulfonamide molecule determines the existence of two different diastereomeric forms (individual and crystal solvate ones) occurring in the corresponding crystals. Molecular structures of the diastereomeric forms in crystals and in the gas phase are studied using quantum chemical calculations. It is established that the inversion of the nitrogen configuration leads to the electron density redistribution and to some geometry shifts in the heteroatom-bearing key fragment of the molecule.







Similar content being viewed by others
REFERENCES
R. J. Davey and J. Garside. From Molecules to Crystallizers: An Introduction to Crystallization. Oxford, England: Oxford University Press, 2000.
J. Bernstein. Polymorphism in Molecular Crystals. Oxford, England: Oxford University Press, 2002.
Crystallization: Basic Concepts and Industrial Applications / Ed. W. eckmann. Wiley, 2013. https://doi.org/10.1002/9783527650323
T. L. Threlfall. Analysis of organic polymorphs. A review. Analyst, 1995, 120(10), 2435. https://doi.org/10.1039/an9952002435
E. H. Lee. A practical guide to pharmaceutical polymorph screening & selection. Asian J. Pharm. Sci., 2014, 9(4), 163-175. https://doi.org/10.1016/j.ajps.2014.05.002
A. Nangia. Conformational polymorphism in organic crystals. Acc. Chem. Res., 2008, 41(5), 595-604. https://doi.org/10.1021/ar700203k
L. Yu. Polymorphism in molecular solids: an extraordinary system of red, orange, and yellow crystals. Acc. Chem. Res., 2010, 43(9), 1257-1266. https://doi.org/10.1021/ar100040r
C. Döring, C. Näther, I. Jess, K. Ibrom, and P. G. Jones. Two polymorphs of 4-hydroxypiperidine with different NH configurations. CrystEngComm, 2015, 17(28), 5206-5215. https://doi.org/10.1039/c4ce02477j
O. A. Lodochnikova, D. P. Gerasimova, and V. V. Plemenkov. From classical to supramolecular dynamic stereochemistry: Double crystallization-nduced diastereomerization of thiazine sulfonamide. Chirality, 2021, 33(7), 409-420. https://doi.org/10.1002/chir.23316
O. A. Tevs, Y. V. Veremeichik, D. N. Shurpik, O. A. Lodochnikova, and V. V. Plemenkov. Acylated benzothiazinesulfoneamides: Synthesis and molecular structure. Russ. J. Gen. Chem., 2016, 86(8), 1850-1853. https://doi.org/10.1134/s1070363216080120
G. M. Sheldrick. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053273314026370
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053229614024218
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J .A. K. Howard, and H. Puschmann. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr., 2009, 42(2), 339-341. https://doi.org/10.1107/s0021889808042726
A. L. Spek. Structure validation in chemical crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65(2), 148-155. https://doi.org/10.1107/s090744490804362x
C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler, and P. A. Wood. Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr., 2020, 53(1), 226-235. https://doi.org/10.1107/s1600576719014092
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox. Gaussian16, Revision A.03. Wallingford, CT, USA: Gaussian, 2016.
J.-D. Chai and M. Head-Gordon. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys., 2008, 10(44), 6615. https://doi.org/10.1039/b810189b
R. A. Kendall, T. H. Dunning, and R. J. Harrison. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys., 1992, 96(9), 6796-6806. https://doi.org/10.1063/1.462569
R. F. W. Bader. Atoms in Molecules: A Quantum Theory. New York, USA: Clarendon, 1990.
T. A. Keith. AIMAll (version 19.10.12). Overland Park, KS, USA: TK Gristmill Software, 2010.
S. A. Shteingolts and R. R. Fayzullin. X-ray charge density study of the drug methimazole with Z′ = 2: Differences in the electronic structure of the thiourea core due to crystal packing effects. Cryst. Growth Des., 2020, 20(3), 2074-2090. https://doi.org/10.1021/acs.cgd.9b01715
S. A. Shteingolts, A. F. Saifina, L. F. Saifina, V. E. Semenov, G. K. Fukin, and R. R. Fayzullin. X-ray charge density study of the 6-methyluracil derivative in the crystal: Revealing, consequences, and multipole refinement of minor static disorder. J. Mol. Struct., 2021, 1228, 129724. https://doi.org/10.1016/j.molstruc.2020.129724
S. A. Shteingolts, A. I. Stash, V. G. Tsirelson, and R. R. Fayzullin. Real-space interpretation of interatomic charge transfer and electron exchange effects by combining static and kinetic potentials and associated vector fields. Chem. - Eur. J., 2022, 28(48), e202200985. https://doi.org/10.1002/chem.202200985
S. V. Kartashov, S. A. Shteingolts, A. I. Stash, V. G. Tsirelson, and R. R. Fayzullin. Electronic and crystal packing effects in terms of static and kinetic force field features: Picolinic acid N-oxide and methimazole. Cryst. Growth Des., 2023, 23(3), 1726-1742. https://doi.org/10.1021/acs.cgd.2c01286
A. F. Saifina, S. V. Kartashov, A. I. Stash, V. G. Tsirelson, and R. R. Fayzullin. Unified picture of interatomic interactions, structures, and chemical reactions by means of electrostatic and kinetic force density fields: Appel′s salt and its ion pairs. Cryst. Growth Des., 2023, 23(4), 3002-3018. https://doi.org/10.1021/acs.cgd.3c00088
C. Silva Lopez and A. R. de Lera. Bond ellipticity as a measure of electron delocalization in structure and reactivity. Curr. Org. Chem., 2011, 15(20), 3576-3593. https://doi.org/10.2174/138527211797636228
Funding
The XRD experiment and the quantum chemical calculations were funded by the Russian Science Foundation (project No. 22-13-00284) at the Distributed Spectral-Analytical Center of Shared Facilities for the Study of Structure, Composition and Properties of Substances and Materials of FRC Kazan Scientific Center of RAS. The synthesis of the key compound was partially funded by the Priority 2030 Strategic Leadership Program of KFU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 8, 114890.https://doi.org/10.26902/JSC_id114890
Rights and permissions
About this article
Cite this article
Gerasimova, D.P., Veremeichik, Y.V. & Lodochnikova, O.A. Two Crystal Structures of Benzo-1,2-Thiazine-S,S-Dioxide with a Condensed Norbornane Fragment Differing in the Configuration of the Nitrogen Atom in the Molecule. J Struct Chem 64, 1504–1512 (2023). https://doi.org/10.1134/S0022476623080140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623080140