Abstract
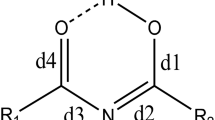
In the current research, the origin of conformational preferences in trifluoro-acetylacetaldehyde (TFAAD) by detail analysis of the intramolecular hydrogen bond (IMHB) and π-electron delocalization (π-ED) was investigated. In this regard, all of the possible conformations of keto and enol tautomers of TFAAD at various theoretical levels were optimized. Our results reveal that the chelated enol forms (E11, E21) have extra stability with respect to the other conformations, and at all of the computational levels E21 is more stable than the E11 and identifies as the global minimum. We expected that the extra stability of E21 is probably related to the stronger intramolecular hydrogen bond (IMHB), but quantum chemical calculations confirm the stronger IMHB of E11. Since, the chelated forms are resonance assisted hydrogen bond (RAHB) systems, it seems that the π-ED concept can probably justify this duality. The significance of π-ED by the geometrical factor of Gilli (λ) and the harmonic oscillator model of aromaticity (HOMA) were assessed. According to these indicators, E21 has higher π-ED than E11, in excellent agreement with their stability order. Finally, the population analysis by the natural bond orbital method and atoms in molecules theory were carried out. The stabilization charge transfer energies of RAHB systems also indicate more π- ED in E21. Consequently the π-electron delocalization effect is the superior factor determining the global minimum. The IMHB of the chelated forms, in the first singlet excited state, were also studied. Surprisingly, the results show that the IMHB in E21 is stronger than in E11, in contrast to the ground state.
Similar content being viewed by others
References
G. A. Jefferey, Introduction to Hydrogen Bonding, University Press, Oxford, New York (1997).
G. Gilli and V. Bertolasi, the Chemistry of Enols, Rappoport ZEd, Wiley, Chichester UK (1990).
G. Gilli and P. Gilli, The Nature of Hydrogen Bond, University Press, Oxford (2009).
V. Bertolasi, P. Gilli, V. Ferretti, and G. Gilli, J. Am. Chem. Soc., 113, 4917–4925 (1991).
M. Schröder, F. Gatti, and H. D. Meyer, J. Chem. Phys., 134, 234307 (2011).
M. Aschi, M. D’Abramo, F. Ramondo, I. Daidone, M. D’Alessandro, A. D. Nola, and A. Amadei, J. Phys. Org. Chem., 19, 518–553 (2006).
A. Nowroozi, S. F. Tayyari, and H. Rahemi, Spectrochim. Acta, 59A, 1757 (2003).
A. Nowroozi and H. J. Raissi, Mol. Struct. (THEOCHEM), 759, 93–100 (2006).
G. V. Mil’nikov, et al., J. Chem. Phys., 119, No. 1, 10–13 (2003).
V. B. Delchev and G. S. Nikolov, Monatsh. Chem., 131, No. 2, 107–115 (2000).
M. D. Coutinho-Neto, A. Viel, and U. Manthe, J. Chem. Phys., 121, No. 19, 9207–9210 (2004).
X. Xu, J. Zheng, and D. G. Truhlar, J. Am. Chem. Soc., 137, No. 25, 8026–8029 (2015).
G. A. Pitsevich, A. E. Malevich, E. N. Kozlovskaya, I. Y. Doroshenko, V. E. Pogorelov, V. Sablinskas, and V. Balevicius, Spectrochim. Acta, 145A, 384–393 (2015).
E. Nakhaei and A. Nowroozi, J. Comput. Theor. Chem., 1096, 27–32 (2016).
V. V. Sliznev, S. B. Lapshina, and G. V. Girichev, J. Struct. Chem., 43, 47–55 (2002).
V. V. Sliznev, S. B. Lapshina, and G. V. Girichev, J. Struct. Chem., 47, 220–231 (2006).
H. Azizi Toupkanloo and S. F. Tayyari, J. Struct. Chem., 57, 65–75 (2016).
A. E. Reed, L. A. Curtis, and F. A. Weinhold, Chem. Rev., 88, 899–926 (1988).
R. F. W. Bader, Atoms in Molecules, A Quantum Theory, Clarendon, Oxford, UK (1990).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Jr. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03 revision C 02, Gaussian Inc, Pittsburgh (2003).
F. Biegler-König, J. Schönbohm, and D. Bayles, J. Comp. Chem., 22, 545–559 (2001).
D. E. Glendening, A. E. Reed, and J. E. Carpenter, Weinhold, F. NBO, Version 3.1.
J. Kruszewski and T. M. Krygowski, Bull. Acad. Pol. Sci. Chim., 20, 907–915 (1972).
J. Kruszewski and T. M. Krygowski, Tetrahedron Lett., 3839–3842 (1972).
P. Shuster and G. Zundel, The Hydrogen Bond. Recent Development in Theory and Experiment, Nourth-Holland, Amesterdam (1976).
I. Rozas, I. Alkorta, and J. Elguero, J. Phys. Chem. A, 105, 10462–10467 (2001).
M. Jablonski, A. Kaczmarek, and A. J. Sadlej, J. Phys. Chem. A, 110, 10890–10898 (2006).
A. Nowroozi, H. Raissi, and F. Farzad, J. Mol. Struct. (THEOCHEM), 730, 161–169 (2005).
G. Buemi and F. Zuccarello, Chem. Phys., 306, 115–129 (2004).
E. Espinosa and E. Molins, J. Chem. Phys., 113, 5686–5694 (2000).
A. Nowroozi, H. Hajiabadi, and F. Akbari, Struct. Chem., 25, 251–258 (2014).
P. L. A. Popelier and R. F. W. Bader, J. Phys. Chem., 189, 542–548 (1992).
I. Rozas, I. Alkorta, and J. Elguero, J. Phys. Chem. A, 101, 9457–9463 (1997).
B. A. Shainyan, N. N. Chipanina, T. N. Aksamentova, L. P. Oznobikhina, G. N. Rosentsevig, and I. B. Rosentsevig, Tetrahedron, 66, 8551–8556 (2010).
L. Sobczyk, S. J. Grabowski, and T. M. Krygowski, Chem. Rev., 105, 3513–3560 (2005).
J. Poater, M. Duran, M. Solà, and B. Silvi, Chem. Rev., 105, 3911–3947 (2005).
T. M. Krygowski and B. T. Stepień, Chem. Rev., 105, 3482–3512 (2005).
T. M. Krygowski and M. K. Cyranski, Chem. Rev., 101, 1385–142 (2001).
A. Nowroozi, E. Nakhaei, and E. Masumian, Struct. Chem., 25, 1415–1422 (2014).
G. Gilli, F. Bellucci, V. Ferretti, and V. Bertolasi, J. Am. Chem. Soc., 111, 1023–1028 (1989).
T. M. Krygowski and M. K. Cyranski, Tetrahedron, 52, 1713–1722 (1996).
A. Schafer, C. Huber, and R. Ahlrichs, J. Chem. Phys., 100, 5829–5835 (1994).
R. Ahlrichs, M. Bar, M. Haser, H. Horn, and C. Kolmel, Chem. Phys. Lett., 162, 165–169 (1989).
K. Shayan and A. Nowroozi, Struct. Chem., 27, 1769–1780 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Naeini, F.G., Nowroozi, A. Evaluation the origin of conformational preferences in trifluoroacetylacetaldehyde by detail analysis of the intramolecular hydrogen bond and π-electron delocalization in the ground and first excited states. J Struct Chem 58, 1251–1261 (2017). https://doi.org/10.1134/S0022476617060257
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476617060257