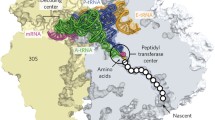
Abstract
Methylation of nucleotides in rRNA is one of the basic mechanisms of bacterial resistance to protein synthesis inhibitors. The genes for corresponding methyltransferases have been found in producer strains and clinical isolates of pathogenic bacteria. In some cases, rRNA methylation by housekee** enzymes is, on the contrary, required for the action of antibiotics. The effects of rRNA modifications associated with antibiotic efficacy may be cooperative or mutually exclusive. Evolutionary relationships between the systems of rRNA modification by housekee** enzymes and antibiotic resistance-related methyltransferases are of particular interest. In this review, we discuss the above topics in detail.




Similar content being viewed by others
Abbreviations
- DC:
-
decoding center
- PET:
-
peptide exit tunnel
- PTC:
-
peptidyl transferase center
- rRNA:
-
ribosomal RNA
REFERENCES
Arenz, S., and Wilson, D. N. (2016) Bacterial protein synthesis as a target for antibiotic inhibition, Cold Spring Harb. Perspect. Med., 6, a025361, doi: https://doi.org/10.1101/cshperspect.a025361.
Sergiev, P. V., Aleksashin, N. A., Chugunova, A. A., Polikanov, Y. S., and Dontsova, O. A. (2018) Structural and evolutionary insights into ribosomal RNA methylation, Nat. Chem. Biol., 14, 226-235.
Bogdanov, A. A., Sumbatyan, N. V., Shishkina, A. V., Karpenko, V. V., and Korshunova, G. A. (2010) Ribosomal tunnel and translation regulation, Biochemistry (Moscow), 75, 1501-1516.
Skinner, R., Cundliffe, E., and Schmidt, F. J. (1983) Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics, J. Biol. Chem., 258, 12702-12706.
Pernodet, J. L., Fish, S., Blondelet-Rouault, M. H., and Cundliffe, E. (1996) The macrolide-lincosamide-streptogramin B resistance phenotypes characterized by using a specifically deleted, antibiotic-sensitive strain of Streptomyces lividans, Antimicrob. Agents Chemother., 40, 581-585.
Roberts, M. C., Sutcliffe, J., Courvalin, P., Jensen, L. B., Rood, J., and Seppala, H. (1999) Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants, Antimicrob. Agents Chemother., 43, 2823-2830.
Arthur, M., Brisson-Noël, A., and Courvalin, P. (1987) Origin and evolution of genes specifying resistance to macrolide, lincosamide and streptogramin antibiotics: data and hypotheses, J. Antimicrob. Chemother., 20, 783-802.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990) Basic local alignment search tool, J. Mol. Biol., 215, 403-410.
Bhujbalrao, R., and Anand, R. (2019) Deciphering determinants in ribosomal methyltransferases that confer antimicrobial resistance, J. Am. Chem. Soc., 141, 1425-1429.
Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C., and Ferrin, T. E. (2004) UCSF Chimera – a visualization system for exploratory research and analysis, J. Comput. Chem., 25, 1605-1612.
The RNAcentral Consortium (2019) RNAcentral: a hub of information for non-coding RNA sequences, Nucleic Acids Res., 47, D221-D229.
Hansen, J. L., Ippolito, J. A., Ban, N., Nissen, P., Moore, P. B., and Steitz, T. A. (2002) The structures of four macrolide antibiotics bound to the large ribosomal subunit, Mol. Cell, 10, 117-128.
Bulkley, D., Innis, C. A., Blaha, G., and Steitz, T. A. (2010) Revisiting the structures of several antibiotics bound to the bacterial ribosome, Proc. Natl. Acad. Sci. USA, 107, 17158-17163.
Svetlov, M. S., Plessa, E., Chen, C.-W., Bougas, A., Krokidis, M. G., Dinos, G. P., and Polikanov, Y. S. (2019) High-resolution crystal structures of ribosome-bound chloramphenicol and erythromycin provide the ultimate basis for their competition, RNA, 25, 600-606.
Gupta, P., Sothiselvam, S., Vázquez-Laslop, N., and Mankin, A. S. (2013) Deregulation of translation due to post-transcriptional modification of rRNA explains why erm genes are inducible, Nat. Commun., 4, 1984.
Subramanian, S. L., Ramu, H., and Mankin, A. S. (2012) Inducible resistance to macrolide antibiotics, in Antibiotic Discovery and Development (Dougherty, T. J., and Pucci, M. J., eds.) Springer US, Boston, MA, pp. 455-484.
Vazquez-Laslop, N., Thum, C., and Mankin, A. S. (2008) Molecular mechanism of drug-dependent ribosome stalling, Mol. Cell, 30, 190-202.
Arenz, S., Ramu, H., Gupta, P., Berninghausen, O., Beckmann, R., Vázquez-Laslop, N., Mankin, A. S., and Wilson, D. N. (2014) Molecular basis for erythromycin-dependent ribosome stalling during translation of the ErmBL leader peptide, Nat. Commun., 5, 3501.
Weisblum, B. (1995) Insights into erythromycin action from studies of its activity as inducer of resistance, Antimicrob. Agents Chemother., 39, 797-805.
Kwak, J. H., Choi, E. C., and Weisblum, B. (1991) Transcriptional attenuation control of ermK, a macrolide-lincosamide-streptogramin B resistance determinant from Bacillus licheniformis, J. Bacteriol., 173, 4725-4735.
Morris, R. P., Nguyen, L., Gatfield, J., Visconti, K., Nguyen, K., et al. (2005) Ancestral antibiotic resistance in Mycobacterium tuberculosis, Proc. Natl. Acad. Sci. USA, 102, 12200-12205.
Gupta, P., Kannan, K., Mankin, A. S., and Vázquez-Laslop, N. (2013) Regulation of gene expression by macrolide-induced ribosomal frameshifting, Mol. Cell, 52, 629-642.
Liu, M., and Douthwaite, S. (2002) Resistance to the macrolide antibiotic tylosin is conferred by single methylations at 23S rRNA nucleotides G748 and A2058 acting in synergy, Proc. Natl. Acad. Sci. USA, 99, 14658-14663.
Liu, M., and Douthwaite, S. (2002) Methylation at nucleotide G745 or G748 in 23S rRNA distinguishes Gram-negative from Gram-positive bacteria, Mol. Microbiol., 44, 195-204.
Yakhnin, H., Yakhnin, A. V., Mouery, B. L., Mandell, Z. F., Karbasiafshar, C., Kashlev, M., and Babitzke, P. (2019) NusG-dependent RNA polymerase pausing and tylosin-Dependent ribosome stalling are required for tylosin resistance by inducing 23S rRNA methylation in Bacillus subtilis, mBio, 10, e02665-19.
Takaya, A., Sato, Y., Shoji, T., and Yamamoto, T. (2013) Methylation of 23S rRNA nucleotide G748 by RlmAII methyltransferase renders Streptococcus pneumoniae telithromycin susceptible, Antimicrob. Agents Chemother., 57, 3789-3796.
Desmolaize, B., Fabret, C., Brégeon, D., Rose, S., Grosjean, H., and Douthwaite, S. (2011) A single methyltransferase YefA (RlmCD) catalyses both m5U747 and m5U1939 modifications in Bacillus subtilis 23S rRNA, Nucleic Acids Res., 39, 9368-9375.
Shoji, T., Takaya, A., Sato, Y., Kimura, S., Suzuki, T., and Yamamoto, T. (2015) RlmCD-mediated U747 methylation promotes efficient G748 methylation by methyltransferase RlmAII in 23S rRNA in Streptococcus pneumoniae; interplay between two rRNA methylations responsible for telithromycin susceptibility, Nucleic Acids Res., 43, 8964-8972.
Long, K. S., Poehlsgaard, J., Kehrenberg, C., Schwarz, S., and Vester, B. (2006) The Cfr rRNA methyltransferase confers resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A antibiotics, Antimicrob. Agents Chemother., 50, 2500-2505.
LaMarre, J. M., Locke, J. B., Shaw, K. J., and Mankin, A. S. (2011) Low fitness cost of the multidrug resistance gene cfr, Antimicrob. Agents Chemother., 55, 3714-3719.
Smith, L. K., and Mankin, A. S. (2008) Transcriptional and translational control of the mlr operon, which confers resistance to seven classes of protein synthesis inhibitors, Antimicrob. Agents Chemother., 52, 1703-1712.
Toh, S.-M., **ong, L., Bae, T., and Mankin, A. S. (2008) The methyltransferase YfgB/RlmN is responsible for modification of adenosine 2503 in 23S rRNA, RNA, 14, 98-106.
LaMarre, J. M., Howden, B. P., and Mankin, A. S. (2011) Inactivation of the indigenous methyltransferase RlmN in Staphylococcus aureus increases linezolid resistance, Antimicrob. Agents Chemother., 55, 2989-2991.
Pletnev, P., Guseva, E., Zanina, A., Evfratov, S., Dzama, M., et al. (2020) Comprehensive functional analysis of Escherichia coli ribosomal RNA methyltransferases, Front. Genet., 11, 97.
Vázquez-Laslop, N., Ramu, H., Klepacki, D., Kannan, K., and Mankin, A. S. (2010) The key function of a conserved and modified rRNA residue in the ribosomal response to the nascent peptide, EMBO J., 29, 3108-3117.
Kaminska, K. H., Purta, E., Hansen, L. H., Bujnicki, J. M., Vester, B., and Long, K. S. (2010) Insights into the structure, function and evolution of the radical-SAM 23S rRNA methyltransferase Cfr that confers antibiotic resistance in bacteria, Nucleic Acids Res., 38, 1652-1663.
Giessing, A. M. B., Jensen, S. S., Rasmussen, A., Hansen, L. H., Gondela, A., Long, K., Vester, B., and Kirpekar, F. (2009) Identification of 8-methyladenosine as the modification catalyzed by the radical SAM methyltransferase Cfr that confers antibiotic resistance in bacteria, RNA, 15, 327-336.
Yan, F., LaMarre, J. M., Röhrich, R., Wiesner, J., Jomaa, H., Mankin, A. S., and Fujimori, D. G. (2010) RlmN and Cfr are radical SAM enzymes involved in methylation of ribosomal RNA, J. Am. Chem. Soc., 132, 3953-3964.
Atkinson, G. C., Hansen, L. H., Tenson, T., Rasmussen, A., Kirpekar, F., and Vester, B. (2013) Distinction between the Cfr methyltransferase conferring antibiotic resistance and the housekee** RlmN methyltransferase, Antimicrob. Agents Chemother., 57, 4019-4026.
Stojković, V., Noda-Garcia, L., Tawfik, D. S., and Fujimori, D. G. (2016) Antibiotic resistance evolved via inactivation of a ribosomal RNA methylating enzyme, Nucleic Acids Res., 44, 8897-8907.
Benítez-Páez, A., Villarroya, M., and Armengod, M.-E. (2012) The Escherichia coli RlmN methyltransferase is a dual-specificity enzyme that modifies both rRNA and tRNA and controls translational accuracy, RNA, 18, 1783-1795.
Conrad, J., Sun, D., Englund, N., and Ofengand, J. (1998) The rluC gene of Escherichia coli codes for a pseudouridine synthase that is solely responsible for synthesis of pseudouridine at positions 955, 2504, and 2580 in 23 S ribosomal RNA, J. Biol. Chem., 273, 18562-18566.
Toh, S.-M., and Mankin, A. S. (2008) An indigenous posttranscriptional modification in the ribosomal peptidyl transferase center confers resistance to an array of protein synthesis inhibitors, J. Mol. Biol., 380, 593-597.
Davies, J., Gorini, L., and Davis, B. D. (1965) Misreading of RNA codewords induced by aminoglycoside antibiotics, Mol. Pharmacol., 1, 93-106.
Hausner, T. P., Geigenmüller, U., and Nierhaus, K. H. (1988) The allosteric three-site model for the ribosomal elongation cycle. New insights into the inhibition mechanisms of aminoglycosides, thiostrepton, and viomycin, J. Biol. Chem., 263, 13103-13111.
Doi, Y., and Arakawa, Y. (2007) 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides, Clin. Infect. Dis., 45, 88-94.
Grosjean, H. (2009) DNA and RNA Modification Enzymes: Structure, Mechanism, Function and Evolution, Landes Bioscience, Austin, Tex.
Doi, Y., Wachino, J., and Arakawa, Y. (2016) Aminoglycoside resistance, Infect. Dis. Clin. North Am., 30, 523-537.
Wachino, J., Shibayama, K., Kurokawa, H., Kimura, K., Yamane, K., Suzuki, S., Shibata, N., Ike, Y., and Arakawa, Y. (2007) Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides, Antimicrob. Agents Chemother., 51, 4401-4409.
Macmaster, R., Zelinskaya, N., Savic, M., Rankin, C. R., and Conn, G. L. (2010) Structural insights into the function of aminoglycoside-resistance A1408 16S rRNA methyltransferases from antibiotic-producing and human pathogenic bacteria, Nucleic Acids Res., 38, 7791-7799.
Borovinskaya, M. A., Pai, R. D., Zhang, W., Schuwirth, B. S., Holton, J. M., et al. (2007) Structural basis for aminoglycoside inhibition of bacterial ribosome recycling, Nat. Struct. Mol. Biol., 14, 727-732.
Stanley, R. E., Blaha, G., Grodzicki, R. L., Strickler, M. D., and Steitz, T. A. (2010) The structures of the anti-tuberculosis antibiotics viomycin and capreomycin bound to the 70S ribosome, Nat. Struct. Mol. Biol., 17, 289-293.
Selmer, M., Dunham, C. M., Murphy, F. V., Weixlbaumer, A., Petry, S., Kelley, A. C., Weir, J. R., and Ramakrishnan, V. (2006) Structure of the 70S ribosome complexed with mRNA and tRNA, Science, 313, 19351942.
Galimand, M., Schmitt, E., Panvert, M., Desmolaize, B., Douthwaite, S., Mechulam, Y., and Courvalin, P. (2011) Intrinsic resistance to aminoglycosides in Enterococcus faecium is conferred by the 16S rRNA m5C1404-specific methyltransferase EfmM, RNA, 17, 251-262.
Demirci, H., Larsen, L. H. G., Hansen, T., Rasmussen, A., Cadambi, A., Gregory, S. T., Kirpekar, F., and Jogl, G. (2010) Multi-site-specific 16S rRNA methyltransferase RsmF from Thermus thermophilus, RNA, 16, 1584-1596.
François, B., Russell, R. J. M., Murray, J. B., Aboul-ela, F., Masquida, B., Vicens, Q., and Westhof, E. (2005) Crystal structures of complexes between aminoglycosides and decoding A site oligonucleotides: role of the number of rings and positive charges in the specific binding leading to miscoding, Nucleic Acids Res., 33, 5677-5690.
Maus, C. E., Plikaytis, B. B., and Shinnick, T. M. (2005) Mutation of tlyA confers capreomycin resistance in Mycobacterium tuberculosis, Antimicrob. Agents Chemother., 49, 571-577.
Sałamaszyńska-Guz, A., Rose, S., Lykkebo, C. A., Taciak, B., Bącal, P., Uśpieński, T., and Douthwaite, S. (2017) Biofilm formation and motility are promoted by Cj0588-directed methylation of rRNA in Campylobacter jejuni, Front. Cell. Infect. Microbiol., 7, 533.
Johansen, S. K., Maus, C. E., Plikaytis, B. B., and Douthwaite, S. (2006) Capreomycin binds across the ribosomal subunit interface using tlyA-encoded 2′-O-methylations in 16S and 23S rRNAs, Mol. Cell, 23, 173-182.
Ermolenko, D. N., Spiegel, P. C., Majumdar, Z. K., Hickerson, R. P., Clegg, R. M., and Noller, H. F. (2007) The antibiotic viomycin traps the ribosome in an intermediate state of translocation, Nat. Struct. Mol. Biol., 14, 493-497.
Monshupanee, T., Johansen, S. K., Dahlberg, A. E., and Douthwaite, S. (2012) Capreomycin susceptibility is increased by TlyA-directed 2′-O-methylation on both ribosomal subunits, Mol. Microbiol., 85, 1194-1203.
Rahman, A., Srivastava, S. S., Sneh, A., Ahmed, N., and Krishnasastry, M. V. (2010) Molecular characterization of tlyA gene product, Rv1694 of Mycobacterium tuberculosis: a non-conventional hemolysin and a ribosomal RNA methyl transferase, BMC Biochem., 11, 35.
Freihofer, P., Akbergenov, R., Teo, Y., Juskeviciene, R., Andersson, D. I., and Böttger, E. C. (2016) Nonmutational compensation of the fitness cost of antibiotic resistance in mycobacteria by overexpression of tlyA rRNA methylase, RNA, 22, 1836-1843.
Felnagle, E. A., Rondon, M. R., Berti, A. D., Crosby, H. A., and Thomas, M. G. (2007) Identification of the biosynthetic gene cluster and an additional gene for resistance to the antituberculosis drug capreomycin, Appl. Environ. Microbiol., 73, 4162-4170.
Bijpuria, S., Sharma, R., and Taneja, B. (2020) Deletion of RsmE 16S rRNA methyltransferase leads to low level increase in aminoglycoside resistance in Mycobacterium smegmatis, bioRxiv, doi: https://doi.org/10.1101/2020.01.15.907279.
Basturea, G. N., Rudd, K. E., and Deutscher, M. P. (2006) Identification and characterization of RsmE, the founding member of a new RNA base methyltransferase family, RNA, 12, 426-434.
Andersen, N. M., and Douthwaite, S. (2006) YebU is a m5C methyltransferase specific for 16 S rRNA nucleotide 1407, J. Mol. Biol., 359, 777-786.
Gutierrez, B., Escudero, J. A., San Millan, A., Hidalgo, L., Carrilero, L., et al. (2012) Fitness cost and interference of Arm/Rmt aminoglycoside resistance with the RsmF housekee** methyltransferases, Antimicrob. Agents Chemother., 56, 2335-2341.
Lioy, V. S., Goussard, S., Guerineau, V., Yoon, E.-J., Courvalin, P., Galimand, M., and Grillot-Courvalin, C. (2014) Aminoglycoside resistance 16S rRNA methyltransferases block endogenous methylation, affect translation efficiency and fitness of the host, RNA, 20, 382-391.
Kimura, S., and Suzuki, T. (2010) Fine-tuning of the ribosomal decoding center by conserved methyl-modifications in the Escherichia coli 16S rRNA, Nucleic Acids Res., 38, 1341-1352.
Vidučić, D., Obranić, S., Matovina, M., Babić, F., and Vlahoviček, G. M. (2014) Host fitness effects of aminoglycoside resistance 16S rRNA G1405 and A1408 methyltransferases from clinical pathogens and natural antibiotic producers, FEBS J., 281, Suppl. s1, 285.
Thompson, J., Schmidt, F., and Cundliffe, E. (1982) Site of action of a ribosomal RNA methylase conferring resistance to thiostrepton, J. Biol. Chem., 257, 7915-7917.
Lentzen, G., Klinck, R., Matassova, N., Aboul-ela, F., and Murchie, A. I. H. (2003) Structural basis for contrasting activities of ribosome binding thiazole antibiotics, Chem. Biol., 10, 769-778.
Harms, J. M., Wilson, D. N., Schluenzen, F., Connell, S. R., Stachelhaus, T., et al. (2008) Translational regulation via L11: molecular switches on the ribosome turned on and off by thiostrepton and micrococcin, Mol. Cell, 30, 26-38.
Cundliffe, E., and Thompson, J. (1981) The mode of action of nosiheptide (multhiomycin) and the mechanism of resistance in the producing organism, J. Gen. Microbiol., 126, 185-192.
Arenz, S., Juette, M. F., Graf, M., Nguyen, F., Huter, P., Polikanov, Y. S., Blanchard, S. C., and Wilson, D. N. (2016) Structures of the orthosomycin antibiotics avilamycin and evernimicin in complex with the bacterial 70S ribosome, Proc. Natl. Acad. Sci. USA, 113, 7527-7532.
Weitnauer, G., Gaisser, S., Trefzer, A., Stockert, S., Westrich, L., Quiros, L. M., Mendez, C., Salas, J. A., and Bechthold, A. (2001) An ATP-binding cassette transporter and two rRNA methyltransferases are involved in resistance to avilamycin in the producer organism Streptomyces viridochromogenes Tü57, Antimicrob. Agents Chemother., 45, 690-695.
Treede, I., Jakobsen, L., Kirpekar, F., Vester, B., Weitnauer, G., Bechthold, A., and Douthwaite, S. (2003) The avilamycin resistance determinants AviRa and AviRb methylate 23S rRNA at the guanosine 2535 base and the uridine 2479 ribose, Mol. Microbiol., 49, 309-318.
Mann, P. A., **ong, L., Mankin, A. S., Chau, A. S., Mendrick, C. A., et al. (2001) EmtA, a rRNA methyltransferase conferring high-level evernimicin resistance, Mol. Microbiol., 41, 1349-1356.
Ballesta, J. P., and Cundliffe, E. (1991) Site-specific methylation of 16S rRNA caused by pct, a pactamycin resistance determinant from the producing organism, Streptomyces pactum, J. Bacteriol., 173, 7213-7218.
Mankin, A. S. (1997) Pactamycin resistance mutations in functional sites of 16 S rRNA, J. Mol. Biol., 274, 8-15.
Polikanov, Y. S., Osterman, I. A., Szal, T., Tashlitsky, V. N., Serebryakova, M. V., et al. (2014) Amicoumacin a inhibits translation by stabilizing mRNA interaction with the ribosome, Mol. Cell, 56, 531-540.
Lesnyak, D. V., Osipiuk, J., Skarina, T., Sergiev, P. V., Bogdanov, A. A., et al. (2007) Methyltransferase that modifies guanine 966 of the 16 S rRNA: functional identification and tertiary structure, J. Biol. Chem., 282, 5880-5887.
Gu, X. R., Gustafsson, C., Ku, J., Yu, M., and Santi, D. V. (1999) Identification of the 16S rRNA m5C967 methyltransferase from Escherichia coli, Biochemistry, 38, 4053-4057.
Tscherne, J. S., Nurse, K., Popienick, P., Michel, H., Sochacki, M., and Ofengand, J. (1999) Purification, cloning, and characterization of the 16S RNA m5C967 methyltransferase from Escherichia coli, Biochemistry, 38, 1884-1892.
Prokhorova, I. V., Osterman, I. A., Burakovsky, D. E., Serebryakova, M. V., Galyamina, M. A., et al. (2013) Modified nucleotides m(2)G966/m(5)C967 of Escherichia coli 16S rRNA are required for attenuation of tryptophan operon, Sci. Rep., 3, 3236.
Helser, T. L., Davies, J. E., and Dahlberg, J. E. (1972) Mechanism of kasugamycin resistance in Escherichia coli, Nature New Biol., 235, 6-9.
Schluenzen, F., Takemoto, C., Wilson, D. N., Kaminishi, T., Harms, J. M., et al. (2006) The antibiotic kasugamycin mimics mRNA nucleotides to destabilize tRNA binding and inhibit canonical translation initiation, Nat. Struct. Mol. Biol., 13, 871-878.
Vila-Sanjurjo, A., Squires, C. L., and Dahlberg, A. E. (1999) Isolation of kasugamycin resistant mutants in the 16 S ribosomal RNA of Escherichia coli, J. Mol. Biol., 293, 1-8.
Demirci, H., Murphy, F., Belardinelli, R., Kelley, A. C., Ramakrishnan, V., et al. (2010) Modification of 16S ribosomal RNA by the KsgA methyltransferase restructures the 30S subunit to optimize ribosome function, RNA, 16, 2319-2324.
Connolly, K., Rife, J. P., and Culver, G. (2008) Mechanistic insight into the ribosome biogenesis functions of the ancient protein KsgA, Mol. Microbiol., 70, 1062-1075.
Mecsas, J., Bilis, I., and Falkow, S. (2001) Identification of attenuated Yersinia pseudotuberculosis strains and characterization of an orogastric infection in BALB/c mice on day 5 postinfection by signature-tagged mutagenesis, Infect. Immun., 69, 2779-2787.
Wimberly, B. T., Brodersen, D. E., Clemons, W. M., Morgan-Warren, R. J., Carter, A. P., Vonrhein, C., Hartsch, T., and Ramakrishnan, V. (2000) Structure of the 30S ribosomal subunit, Nature, 407, 327-339.
Schuwirth, B. S., Day, J. M., Hau, C. W., Janssen, G. R., Dahlberg, A. E., Cate, J. H. D., and Vila-Sanjurjo, A. (2006) Structural analysis of kasugamycin inhibition of translation, Nat. Struct. Mol. Biol, 13, 879-886.
Demirci, H., Murphy, F. V., Murphy, E. L., Connetti, J. L., Dahlberg, A. E., Jogl, G., and Gregory, S. T. (2014) Structural analysis of base substitutions in Thermus thermophilus 16S rRNA conferring streptomycin resistance, Antimicrob. Agents Chemother., 58, 4308-4317.
Nishimura, K., Hosaka, T., Tokuyama, S., Okamoto, S., and Ochi, K. (2007) Mutations in rsmG, encoding a 16S rRNA methyltransferase, result in low-level streptomycin resistance and antibiotic overproduction in Streptomyces coelicolor A3(2), J. Bacteriol., 189, 3876-3883.
Okamoto, S., Tamaru, A., Nakajima, C., Nishimura, K., Tanaka, Y., Tokuyama, S., Suzuki, Y., and Ochi, K. (2007) Loss of a conserved 7-methylguanosine modification in 16S rRNA confers low-level streptomycin resistance in bacteria, Mol. Microbiol., 63, 1096-1106.
Mikheil, D. M., Shippy, D. C., Eakley, N. M., Okwumabua, O. E., and Fadl, A. A. (2012) Deletion of gene encoding methyltransferase (gidB) confers high-level antimicrobial resistance in Salmonella, J. Antibiot., 65, 185-192.
Carter, A. P., Clemons, W. M., Brodersen, D. E., Morgan-Warren, R. J., Wimberly, B. T., and Ramakrishnan, V. (2000) Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics, Nature, 407, 340-348.
Benítez-Páez, A., Cárdenas-Brito, S., Corredor, M., Villarroya, M., and Armengod, M. E. (2013) Mutaciones en genes modificadores de ARN ribosómico y la resistencia a aminoglucósidos: el caso del gen rsmG, Biomédica, 34, 41, doi: https://doi.org/10.7705/biomedica.v34i0.1702.
Gustafsson, C., and Persson, B. C. (1998) Identification of the rrmA gene encoding the 23S rRNA m1G745 methyltransferase in Escherichia coli and characterization of an m1G745-deficient mutant, J. Bacteriol., 180, 359-365.
Jenner, L., Starosta, A. L., Terry, D. S., Mikolajka, A., Filonava, L., et al. (2013) Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis, Proc. Natl. Acad. Sci. USA, 110, 3812-3816.
Lupien, A., Gingras, H., Leprohon, P., and Ouellette, M. (2015) Induced tigecycline resistance in Streptococcus pneumoniae mutants reveals mutations in ribosomal proteins and rRNA, J. Antimicrob. Chemother., 70, 2973-2980.
Nichols, R. J., Sen, S., Choo, Y. J., Beltrao, P., Zietek, M., et al. (2011) Phenotypic landscape of a bacterial cell, Cell, 144, 143-156.
Funding
This work was financially supported by the Russian Foundation for Basic Research (project nos. 20-04-00736 and 20-54-53014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Osterman, I.A., Dontsova, O.A. & Sergiev, P.V. rRNA Methylation and Antibiotic Resistance. Biochemistry Moscow 85, 1335–1349 (2020). https://doi.org/10.1134/S000629792011005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629792011005X