Abstract
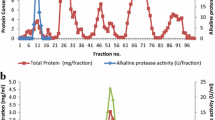
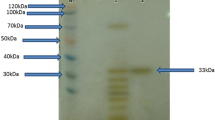
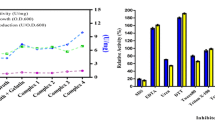
The study describes purification and characterization of two alkaline proteases in comparative manner from haloalkaliphilic bacteria obtained from two different geographical locations. The enzymes from Haloalkaliphilic bacterium D-15-9 (D-15-9) and Oceanobacillus onchorynchii Mi-10-54 (Mi-10-54) purified by ammonium sulfate fractionation and hydrophobic interaction chromatography were characterized for pH, temperature, metal ions and NaCl stability. The apparent molecular weight of the enzymes was 40 and 28 kDa for D-15-9 and Mi-10-54, respectively. Both proteases optimally catalyzed the reactions at 50°C, pH 10.5–10.0 and 0.25–0.5 M NaCl. The Mi-10-54 protease was more thermally stable in comparison to D-15-9 enzyme. While NaCl did not significantly affect the temperature optima of the D-15-9 protease at lower NaCl concentrations, it shifted it from 50 to 80°C in the presence of 3 M NaCl. However, a similar pattern was not evident for the Mi-10-54 protease. On a similar note, there was a shift in the temperature optima from 50 to 60°C in the presence of 10 mM Ca2+ for Mi-10-54 protease, while D-15-9 protease did not display the similar effect. Instead, the D-15-9 protease denatured easily at higher temperatures and Ca2+. Both the enzymes were stable in urea, metal ions, oxidizing and reducing agents and inhibitors. The sensitivity to PMSF suggested that both enzymes were serine proteases. Moderate stability against hydrogen peroxide was also evident for both proteases.












Similar content being viewed by others
REFERENCES
Giménez, M.I., Studdert, C.A., Sánchez, J.J., and De Castro, R.E., Extremophiles, 2000, vol. 4, no. 3, pp. 181–188.
Gerze, A., Omay, D., Guvenilir, Y., Geomicrobiol. J., 2005, vol. 65, no. 1, pp. 40–51.
Kumar, S., Karan, R., Kapoor, S., Singh, S.P., and Khare, S.K., Braz. J. Microbiol., 2012, vol. 43, no. 4, pp. 1595–1603.
Purohit, M.K. and Singh, S.P., Process Biochem., 2014, vol. 49, no. 1, pp. 61–68.
Raval, V.H., Rawal, C.M., Pandey, S., Bhatt, H.B., Dahima, B.R. and Singh, S.P., Ann. Microbiol., 2015, vol. 65, no. 1, pp. 371–381.
Rathore, D.S., Sheikh, M.A., Gohel, S.D., and Singh, S.P., Curr. Microbiol., 2021, vol. 78, no. 4, pp. 1377–1387.
Rathore, D.S. and Singh, S.P., Folia Microbiol., 2021, vol. 66, no. 3, pp. 303–316.
Karan, R., Singh, S.P., Kapoor, S., and Khare, S.K., N. Biotechnol., 2011, vol. 28, no. 2, pp. 136–145.
Rathore, D.S., J. Mar. Biol. Assoc. India, 2019, vol. 61, no. 1, pp. 71–78.
Salwan, R. and Sharma, V., Arch. Microbiol., 2019, vol. 201, no. 7, pp. 863–877.
Bhatt, H.B., Begum, M.A., Chintalapati, S., Chintalapati, V.R., and Singh, S.P., Int. J. Syst. Evol. Microbiol., 2017, vol. 67, no. 11, pp. 4435–4442.
Raval, V.H., Pillai, S., Rawal, C.M., and Singh, S.P., Process Biochem., 2014, vol. 49, no. 6, pp.955–962.
Sharma, A.K., Kikani, B.A., and Singh, S.P., Geomicrobiol. J., 2021, vol. 38, no. 4, pp. 347–364.
Ventosa, A., Nieto, J.J., and Oren, A., Microbiol. Mol. Biol. Rev., 1998, vol. 62, no. 2, pp. 504–544.
Horikoshi, K., Microbiol. Mol. Biol. Rev., 1999, vol. 63, no. 4, pp. 735–750.
Alva, V.A. and Peyton, B.M., Environ. Sci. Technol., 2003, vol. 37, no. 19, pp. 4397–4402.
Studdert, C.A., Castro, R.E., De Seitz, K.H., and Sánchez, J.J., Arch. Microbiol., 1997, vol. 168, no. 6, pp. 532–535.
Fujiwara, N.,Yamamoto, K., and Masui, A., J. Ferment. Bioeng., 1991, vol. 72, no. 4, pp. 306–308.
Mustefa Beyan, S.,Venkatesa Prabhu, S.,Mumecha, T.K., and Gemeda, M.T., Curr. Microbiol., 2021, vol. 78, no. 5, pp. 1823–1834.
Purohit, M.K. and Singh, S.P., Int. J. Biol. Macromol., 2013, vol. 53, pp. 138–143.
Pedersen, N.R., Wimmer, R., Matthiesen, R., Pedersen, L.H., and Gessesse, A., Tetrahedron: Asymmetry, 2003, vol. 14, no. 6, pp. 667–673.
Bennur, T., Kumar, A.R., Zinjarde, S., and Javdekar, V., Elsevier GmbH, 2015, vol. 174, pp. 33–47.
Proteinases and Their Inhibitors, Turk, V. and Vitale, L.J., Eds., Elsevier, 1981, pp. 213–222.
Gessesse, A., Hatti-Kaul, R., Gashe, B.A., and Mattiasson, B.O., Enzyme Microb. Technol., 2003, vol. 32, no. 5, pp. 519–524.
Thumar, J. and Singh, S.P., J. Chromatogr. B, 2007, vol. 854, nos. 1–2, pp. 198–203.
Olivera, N., Sequeiros, C., Siñeriz, F. and Breccia, J.D., World J. Microbiol. Biotechnol., 2006, vol. 22, no. 7, pp. 737–743.
Soliman, A., Int. J. Biotechnol. Wellness Ind., 2013, no. 203, pp. 65–74.
Bhatt, H.B. and Singh, S.P., Front. Microbiol., 2020, vol. 11, p. 941.
Bradford, M.M., Anal. Biochem., 1976, vol. 72, nos. 12, pp. 248–254.
Sinsuwan, S., Rodtong, S., and Yongsawatdigul, J., Food Chem., 2010, vol. 119, no. 2, pp. 573–579.
Laemmli, U.K., Nature, 1970, vol. 227, no. 5259, pp. 680–685.
Kamekura, M. and Seno, Y., Biochem. Cell Biol., 1990, vol. 68, no. 1, pp. 352–359.
Prakash, B., Vidyasagar, M., Madhukumar, M.S., Muralikrishna, G., and Sreeramulu, K., Process Biochem., 2009, vol. 44, no. 2, pp. 210–215.
Dodia, M.S., Bhimani, H.G., Rawal, C.M., Joshi, R.H., and Singh, S.P., Bioresour. Technol., 2008, vol. 99, no. 14, pp. 6223–6227.
ACKNOWLEDGMENTS
VHR is grateful for the JRF and SRF Fellowship under the DBT Multi-Institutional Project. The majority of the research work included in this report was carried as part of the DBT Multi-Institutional Project involving Prof. S. K. Khare (IIT Delhi) and Dr. Sanjay Kapoor (University of Delhi South Campus, New Delhi). SPS acknowledges DST-SERB International Travel Fellowships to present his work in Hamburg (Germany), Cape Town (South Africa) and Kyoto (Japan). SPS also acknowledge award of UGC BSR Faculty Fellowship. VHR acknowledges SERB- Young Scientist Award and DST-SERB International Travel Fellowship. Facilities and infrastructure created under UGC-CAS Program, MoES Project and DST-FIST are duly acknowledged.
Availability of data and materials. All data and materials generated are included in this published article with the availability.
Author information
Authors and Affiliations
Contributions
V.H. Raval has carried out the experiment. D.S. Rathore has framed the manuscript and S.P. Singh has designed the experiment, critical suggestions and examination of manuscript.
Corresponding author
Ethics declarations
Ethical approval. This paper does not involve any human participants and animals performed by any of the authors.
Competing interests. The authors declare that they have no conflict of interest in the publication.
Supplementary Information
Rights and permissions
About this article
Cite this article
Raval, V.H., Rathore, D.S. & Singh, S.P. Comparative Studies of the Characteristics of Two Alkaline Proteases from Haloalkaliphilic bacterium D-15-9 and Oceanobacillus onchorynchii Mi-10-54. Appl Biochem Microbiol 58, 551–563 (2022). https://doi.org/10.1134/S0003683822050131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822050131