Abstract
The one-step synthesis method of nitrogen doped microporous carbon monoliths derived from biomass with high-efficiency is developed using a novel ammonia (NH3)-assisted activation process, where NH3 serves as both activating agent and nitrogen source. Both pore forming and nitrogen do** simultaneously proceed during the process, obviously superior to conventional chemical activation. The as-prepared nitrogen-doped active carbons exhibit rich micropores with high surface area and high nitrogen content. Synergetic effects of its high surface area, microporous structure and high nitrogen content, especially rich nitrogen-containing groups for effective CO2 capture (i.e., phenyl amine and pyridine-nitrogen) lead to superior CO2/N2 selectivity up to 82, which is the highest among known nanoporous carbons. In addition, the resulting nitrogen-doped active carbons can be easily regenerated under mild conditions. Considering the outstanding CO2 capture performance, low production cost, simple synthesis procedure and easy scalability, the resulting nitrogen-doped microporous carbon monoliths are promising candidates for selective capture of CO2 in industrial applications.
Similar content being viewed by others
Introduction
Carbon capture and sequestration are effective solution for reducing anthropogenic CO2 emission1,2. Since combustion streams, such as flue gas emitted from coal-fired power plants, may comprise ~70% of N2 and 3–15% of CO2, selective capture of CO2 is highly desired3. Compared with the conventional amine-scrubbing and pressure-swing adsorption technologies1,2, selective physisorption techniques are more effective and environmentally friendly. To date, various porous solids, including porous carbons4,5,6,7,8,9,10,11, metal-organic frameworks (MOFs)12,13,14 and covalent organic frameworks (COFs)15,16 have been extensively studied. Numerous efforts have been focused on optimizing the surface area and pore structure, as well as enhancing their affinity to CO2 by incorporating various functional groups4,5,6,7,8,9,10,11,12,13,14,15,16. Various materials with selective CO2 capture capability have been prepared in laboratory scale; large-scale, low-cost and facile synthesis of materials for effective CO2 capture, however, remains challenging.
Chemical activation of biomass has been broadly adopted for large-scale and low-cost production of porous carbon materials for a wide range of applications17,18,19. However, such biomass-based carbons generally do not possess sufficient capability for selective adsorption of CO219. It has been found that incorporating nitrogen (N)-containing groups into carbons (e.g., phenyl amine (Ph-NH2) and pyridine-N groups) can effectively improve their selective CO2 capture over N2 or CH4, mainly due to the preferred interactions between CO2 and the electronegative N-containing groups4,5,6,7,8,9,10,11. Generally, the do** is achieved by direct activation of N-containing biomass20,21,22,23 or by treating carbons with ammonia24,25. For the former approach, the N-containing moieties, however, are generally volatized during the activation process, resulting in carbons with low N content (e.g., <5 wt%). Similarly, the post-treatment process generally leads to carbons with low N content (e.g., <3 wt%) due to low reaction efficiency between the ammonia and the carbon scaffolds.
We report herein a novel synthesis of N-doped microporous carbon monoliths derived from biomass (corncob) using an ammonia gas (NH3)-assisted activation process, where NH3 serves as both the activating agent and the N source. As shown in Fig. 1, corncob particles were efficiently transformed to N-doped microporous carbon monoliths by one-step NH3-assisted activation process. Both pore forming and nitrogen do** simultaneously proceed during the process, obviously superior to conventional chemical activation. The dual role of NH3 as the activating agent and N source leads to high surface area, superior pore texture and high N content of the as-prepared carbon materials. To the best of our knowledge, such an NH3-assisted activation process with high-efficiency has not been reported yet. The resulting N-doped microporous carbon monoliths exhibit excellent selective CO2 capture performance with excellent CO2 selectivity over N2 of 82, which is the highest among reported nanoporous carbons.
Materials and Methods
Sample preparation
Nitrogen-doped active carbons were prepared by a novel chemical activation method using biomass corncob as the carbon source and NH3 as the activating agent and nitrogen source. Detailed procedures are described as follows. Firstly, after drying for 12 h at 120 °C, corncobs were grounded and sieved into powders with typical size of less than 880 μm. Secondly, the corncob powders were transferred to ceramic boats and heated to 400 °C at 5 °C min−1 under N2 flow of 1.5 L min−1 in a horizontal tube furnace to obtain the carbonized particles. Then N2 was switched to NH3 and the sample was continued to be heated at 400–800 °C under NH3 flow of 1.5 L min−1. Then NH3 was switched back to N2 when activation was completed and temperature was reduced to 400 °C. Finally, sample was obtained by lowering the temperature to room temperature under N2 atmosphere. The resulting N-doped active carbons are denoted as NAC-x-y, where x is the activation temperature (°C) and y is the activation time (hours) used, respectively.
Corncob-derived activated carbons (CACs) were prepared by KOH chemical activation with biomass corncob as carbon sources and KOH as the activating agent. Detailed procedures are described according to previous reports17.
Materials characterization and analysis methods
The textural properties of the samples were performed by N2 sorption at 77 K using a Micromeritics ASAP2020 over a wide relative pressure ranging from about 10−6 to 1.0. Prior to the measurements, all samples were degassed at 300 °C for 10 h. The specific surface area (SSA) was assessed by standard BET method (software available in the ASAP2020) using adsorption data in the relative pressure ranging from 0.02 to 0.25. The total pore volume (Vt) was calculated by converting the amount of N2 adsorbed at a relative pressure of 0.98 to the volume of liquid adsorbate. The micropore volume was calculated by the Dubinine Radushkevich (DR) equation. Pore size distributions (PSDs) were calculated by using the Density Functional Theory (DFT) Plus Software (provided by Micromeritics Instrument Corporation), which is based on calculated adsorption isotherms for pores of different sizes. In the DFT calculations, the equilibrium model of carbon slit-shaped pores with N2 sorption was applied.
Scanning electron microscope (SEM) and Energy Dispersive Spectrometer (EDS) map** images were performed on a FEI NOVA Nano electron microscope. Transmission electron microscopy (TEM) and selected area electron diffraction (SAED) were carried out using JEOL JEM-2100F electron microscope. Elemental analysis was obtained by a Thermo Flash EA2000 elemental analyzer. Fourier transform infrared (FT-IR) spectroscopy for samples was analyzed through a BRUKER EQUI NO -XSS spectrometer using the attenuated total reflectance method. X-ray photoelectron spectroscopy (XPS) analysis was performed with an ESCALAB 250** images of typical sample NAC-800-3; (d) N2 sorption isotherms and (e) Pore size distributions of all NAC samples.
Figure 2c further shows a SEM image of a NAC sample along with its element map**. Noticeably, N is homogenously distributed within the carbon framework. Elemental analysis (Table 1) shows negligible amount of N in natural corncob and its directly carbonized product. Meanwhile, activation in NH3 can easily dope N into carbon framework even at 400 °C, although it is difficult to form a porous structure at such a low temperature. Further increase of the activation temperature leads to increase of N content, reaching ~12 wt% at 800 °C, which is unsurpassable for all existing chemical activation methods (e.g., 5.11 at% for HHC21; 4.84 wt% for PA-400-KOH-1-60023; 8.1 wt% for CN70024; 9.2 wt% for CNO30026; 4.5 wt% for AN27).
Such high N content is comparable with those made from specially designed compounds that require highly complex chemical synthesis (e.g., 10.14 wt% for CP-2-6005; 12.9 wt% for IBN9-NCI-A8; 11.95 wt% for PPN-6-CH2DETA14). The increasing trend of N do** with increasing temperature is different from literatures, where the content of N do** generally decreases with increasing temperature, particularly, when temperature is above 500 °C due to high volatility of the N-containing species6,28. The continuous increase of the N content implies a dynamic balance of N do** and removal during the reactions, where the rate of N do** into carbon is over that of N removal from carbon. This method provides a direct approach to fabricate highly N-doped carbons simply by activating biomass in NH3 atmosphere.
Figure 2d shows N2 sorption isotherms of the samples prepared at different activation temperature and time, which exhibit type I isotherms with typical microporous structure20. With increasing the activation temperature from 400 to 800 °C, isotherms with significantly increased pore volume accompanied with enlarged pore sizes are obtained. As shown in Table 1, NAC-400-2, NAC-500-2, and NAC-600-2 show insignificant value of specific surface area (SSA), implying a poor activation at temperature below 600 °C. SSA gradually increases with increasing activation temperature and time, reaching a maximum of 1154 m2 g−1 with an activation temperature of 800 °C for 3 hours (NAC-800-3). Figure 2e further shows the pore size distributions calculated based on DFT model, which shows enlarged pore diameter and broadened distribution along with increasing activation temperature and time. The pore size distribution of NAC-800-4 is similar to porous carbons prepared by KOH activation17,18,19. These samples contain a large fraction of micropores ranging from 0.8 to 1 nm in diameter, as well as mesopores below 4 nm. Combining with the macroporous structure, such carbons with high-level of N-do** and hierarchically porous structure are of great interest for sorption and other applications.
The nature of N within the carbons is investigated by Fourier transform infrared (FT-IR) spectra and X-ray photoelectron spectroscopy (XPS). FT-IR spectra (Fig. 3a) of samples shows a board band at 2000 cm−1, which is characteristic of the nitrile group or C=N from pyridine and quinoline29,30. The bands at ca. 1590 cm−1 can be assigned to the presence of N-H in-plane deformation vibration or C=C stretching vibration from aromatic rings6. The peaks around 1150–900 cm−1 are attributed to the C-N stretching vibration. The broad weak bands between 950 and 650 cm−1 correspond to out-of-plane N-H deformation vibration5,6. Hence, FT-IR analysis confirms the existence of numerous N-containing groups in the carbon samples.
Besides, XPS spectra of NACs (Fig. 3b) clearly shows the incorporation of N atoms into carbon framework. The bonding configurations of N atoms are further characterized by the high-resolution N1s spectra (Fig. 3c). The peaks of N1s spectra can be deconvolved and assigned to various N-containing groups, including pyridine N (398.1 eV), Ph-NH2 (399.4 eV), pyrrolic N (400.5 eV), quaternary N (401.3 eV) and N-oxides (402–405 eV)5,6,7,8,31. As shown in Fig. 3c, N-containing species within the samples change significantly with activation temperature and time. Specifically, only one peak corresponding to Ph-NH2 group (399.4 eV) can be seen at a low activation temperature (NAC-400-2). Two intense peaks at 398.3 and 399.7 eV can be distinguished above 600 °C, corresponding to pyridine-N and Ph-NH2 respectively. During the activation at 750 °C, multiple N species are formed, including pyridine-N, Ph-NH2, pyrrolic-N, quaternary-N and N-oxides. However, most of N-containing groups are highly volatile at high temperature. With further increased temperature and reaction time, only two N-containing groups, i.e., pyridine-N and Ph-NH2 remain at 800 °C (Figure S1). Noticeably, Ph-NH2 and pyridine-N are the most effective N-containing groups for CO2 capture due to the preferred interactions between CO2 and the electronegative N-containing groups4,5,6,7,8,9,10,11.
From the thermodynamic point of view, the direct reaction between carbon and NH3 (Eq. 2),

is favorable only at high temperature (e.g., △H = 188.53 kJ mol−1 at 1273 K)32,33. The ability to realize high-level of N-do** at low temperature can be attributed to the unique chemical composition of the corncob precursor. The main compositions of corncob are cellulose, hemicellulose and lignin34,35, possessing multiple oxygen (O)-containing groups, e.g., C–O, C=O and –OH etc., which can be identified at 900–1800 cm−1 in the FT-IR spectrums (Figure S2). Such groups may play important roles for N do** during NH3 treatment24,27. As shown in Table 1, large amounts of the O-containing groups are consumed during the initial activation process, as suggested by the obvious decrease of the O content. It is possible that NH3 reacts with such groups, forming amine-containing sites such as Ph-NH2 moieties. With increase of the activation temperature, the carbon scaffolds react with NH3, forming pores by transforming carbon to Hydrogen cyanide gas (HCN)32,33, during which N atoms are also doped into the aromatic rings (e.g., in the forms of C–N or C=N). As a result, the N content continually increases, accompanied by the decrease content of carbon with increasing the degree of the activation.
To confirm the role of the O-containing groups in the do** process, corncob-derived activated carbon (denoted as CAC, SSA of ~3711 m2g−1) is also prepared by the conventional KOH activation method (see preparation details in Experimental Section). Such carbon materials contain significantly lower content of the O-containing groups (~ 7% vs ~20% for the pre-carbonated corncob particles, see Table 1 and Figure S2). Consistently, treating the CAC by NH3 under the same condition leads to a much lower N do** (<2%) even at 800 °C, confirming the essential roles of the O-containing groups in the N-do** process. Based on the studies presented, it is reasonable to conclude that NH3 plays dual roles as the activating agent and N-do** source. During the activation process, such N-containing groups are dynamically generated and removed, leading to the formation of highly porous carbons with high content of Ph-NH2 and pyridine-N groups.
Figure 4a shows the CO2 adsorption performance of as-prepared samples at 1 bar and 298 K. Remarkably, CO2 adsorption capacity improves with increased activation temperature and slightly increased reaction time, reaching a maximum value of 2.81 mmol g−1 for NAC-800-3 due to the high SSA and large amount of N-containing groups (i.e. Ph-NH2 and pyridine-N). For comparison, CAC is also tested for CO2 adsorption. Interestingly, although NAC-800-3 only holds one third of SSA of CAC, its CO2 adsorption capacity is about 33% higher, which reveals the crucial role of N-containing groups on CO2 capture.
(a) CO2 adsorption isotherms of all NAC samples at 1 bar and 298 K; (b) Adsorption isotherms of NAC-800-3 for CO2 at 273 and 298 K, and N2 at 298 K; (c) Adsorption isotherms of CAC for CO2 at 273 and 298 K, and N2 at 298 K; (d) Comparison of CO2 adsorption capacity (298 K, 0.1 bar) and CO2/N2 selectivity of NAC-800-3 with different types of representative solid physisorbents (carbons4,5,6,7,8,9,10,11, MOFs12,13,14, COFs15,16).
More importantly, the N-containing groups also endow NACs with advantages on CO2/N2 selectivity. Adsorption selectivity of CO2 over N2 is calculated by the ratio of initial slopes of the CO2 and N2 adsorption isotherms (Figure S3 and Figure S4). As a result, the CO2/N2 selectivity of representative sample NAC-800-3 is about 82:1 at 298 K. To the best of our knowledge, such CO2/N2 selectivity is one of the highest values among reported nanoporous carbons (e.g., 28 for HCM-DAH-14, 42 for IBN9-NCI8, 59 for CPC 5509, 124 for SU-MAC-50036). In addition, the ideal adsorption solution theory (IAST) selectivity (assuming gas mixture of CO2/N2 with 10% CO2 at 1 bar) is calculated to be 45:1 at 298 K. The value discrepancy between the initial slope method and IAST method can be attributed to the adsorption site heterogeneity37. As shown in Fig. 4d, although higher CO2/N2 selectivity has been achieved on other porous framework materials (e.g., 140 for SIFSIX-Cu-I at 298 K13, 442 for PPN6-CH2DETA at 298 K14, 288 for azo-COP at 323 K15), considering the low-cost, easy scalability and facile synthesis method, the NACs reported in this work are more attractive for potential industrial applications.
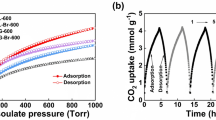
Superior CO2/N2 selectivity of NAC-800-3 is also supported by ultra-high isosteric heats of adsorption (Qst). Qst can be calculated via the CO2 adsorption isotherms at 273 and 298 K (Fig. 4b,c) and applying the Clausius-Clapeyron equation (E.g. 1, see calculation details in the Experimental Section). NAC-800-3 exhibits an ultra-high Qst of 55.1 kJ mol−1 at the initial stage and 30.5 kJ mol−1 at the steady stage (Fig. 5a), which is much higher than those previously reported values of porous carbons (e.g., 35.9–21.1 kJ mol-1 for HCM-DAH-14, 44.1–27.0 kJ mol−1 for IBN9-NCI8). As a comparison, N-free CAC exhibits a lower and almost unchanged Qst of 28.9–24.4 kJ mol−1. The ultra-high Qst of NAC-800-3 at the initial stage decreases gradually at higher uptake, which indicates the presence of active sites (i.e., N-containing groups) on the surface of NAC38.
Overall, both surface chemical property (i.e. N-containing groups) and high SSA accompanied with microporous structure are critically important for selective adsorption of target gas molecules. Although CAC is essentially microporous and possesses ultra-high SSA, it only exhibits low selectivity of about 7:1 (Fig. 4c and Figure S4), which is obviously inferior to NAC-800-3. Hence, merely increasing SSA of carbon adsorbent is not enough for enhancing selective CO2 capture performance. The ultra-high Qst of NAC-800-3 is due to the synergetic effects of high SSA with microporous structure and rich N-containing groups, leading to superior selective adsorption of CO2 over N2.
The reversibility of CO2 adsorption for NAC-800-3 at 298 K has been tested over 3 cycles (Fig. 5b). Adsorption capacities for 3 cycles are almost identical, with generally overlapped desorption and adsorption curves. Thus, CO2 capture in NAC is highly reversible and primarily based on physisorption. The easy regeneration of NAC under mild conditions makes it superior to aqueous amine and amine-modified solids, which require large amount of energy during the regeneration process1,2.