Abstract
Human telomerase reverse transcriptase is a ribonucleoprotein that synthesises telomeric sequences, which decrease at each cell division. In cancer cells, its activity is linked to telomere maintenance leading to unlimited cellular proliferation and immortality. To evaluate the prognostic value of the catalytic subunit telomerase reverse transcriptase (hTERT), we analysed its expression by immunohistochemistry in 122 formalin-fixed lung tumours including 42 squamous cell carcinoma (SCC), 43 adenocarcinoma (ADC), 19 basaloid carcinoma (BC) and 18 small-cell lung carcinoma (SCLC) in comparison with detection of hTERT mRNA by in situ hybridisation and relative telomerase activity by TRAP assay in a subset of tumours. We observed a high concordance between hTERT protein expression and detection of hTERT mRNA and telomerase activity. Telomerase expression varied according to histology (P=0.0002) being significantly lower in ADC than in SCC, BC and SCLC (P<0.0001). Adenocarcinoma and SCC exhibited either a nuclear or a nucleolar staining in contrast with a diffuse nuclear staining observed in most BC and all SCLC (P=0.01). In stage I NSCLC telomerase expression was lower than in other stages (P=0.04), and a nucleolar staining was correlated with a short survival (P=0.03). We concluded that telomerase expression and pattern are distinctive among histopathological classes of lung cancer and convey prognostic influence.
Similar content being viewed by others
Main
Lung cancer represents the leading cause of cancer-related death in industrial countries and comprises about 20% small-cell lung carcinoma (SCLC) and 80% of non-small-cell lung carcinoma (NSCLC). According to the new WHO histological classification (Travis et al, 1999), non-small-cell lung carcinoma include with the common types squamous cell carcinoma (SCC) and adenocarcinoma (ADC), a recently described entity, the basaloid carcinoma (BC), as a variant of large cell carcinoma undergoing a particularly poor outcome (Brambilla et al, 1992). Surgical resection at early-stage disease represents the treatment of choice for NSCLC. However, survival rates remain low fostering identification of new prognostic factors and therapeutic target such as telomerase with the aim of deciphering new modes of adjuvant therapies.
Telomeres, which represent the end of the eukaryotic chromosomes, shorten at each cell division because of incomplete replication by DNA polymerase (Henderson, 1995). This results in telomere shortening leading to chromosome degradation or end fusion and cellular senescence acting as a ‘mitotic clock’ (Harley et al, 1990; Hastie et al, 1990; Allsopp et al, 1992). In germ line cells as well as in tumour cells, telomerase, a ribonucleoprotein complex composed of a reverse transcriptase catalytic subunit (hTERT) that copies a template region of RNA subunit (hTERC), can synthesise telomeric DNA, therefore, allowing cells to proliferate indefinitely (Counter et al, 1992; Nakamura and Cech, 1998; Holt and Shay 1999). While both hTERC and hTERT are required for telomerase activity, hTERC is expressed rather ubiquitously, whereas hTERT is the only limiting factor since its expression is confined to cells expressing telomerase activity (Meyerson et al, 1997; Nakamura et al, 1997; Kolquist et al, 1998).
Telomerase activity (TA) evaluated by a sensitive PCR-based telomere repeat amplification protocol (TRAP) assay has been widely reported in various malignancies such as liver, colorectal, brain, prostate and breast cancers as well as leukaemia (Bacchetti and Counter, 1995; Counter et al, 1995; Langford et al, 1995; Carey et al, 1998; Tahara et al, 1999; Kawakami et al, 2000). Regarding malignancies arising in the thorax, several studies have demonstrated a telomerase activity in SCLC and NSCLC carcinomas including NE tumours and adenocarcinomas and their precursor lesion (namely atypical alveolar hyperplasia) as well as in pulmonary sarcomas and mesotheliomas (Hiyama K et al, 1995; Ahrendt et al, 1997; Yashima et al, 1997; Gomez-Roman et al, 2000; Kumaki et al, 2001, 2002; Nakanishi et al, 2002). Almost all SCLC and the majority of NSCLC display a substantial telomerase activity in 62–96% of the cases (Hiyama K et al, 1995; Albanell et al, 1997; Gomez-Roman et al, 2000). Since a close relationship has been demonstrated between elevated telomerase activity and a poor prognosis in neuroblastoma and gastric carcinoma (Hiyama E et al, 1995a, 1995b), several reports have also suggested that high TA or high hTERT mRNA levels should be correlated with a poor survival in stage I NSCLC (Marchetti et al, 1999, 2002; Wang et al, 2002). High levels of telomerase have also been associated with tumour recurrence, histological type, grade (Marchetti et al, 1999, 2002; Kumaki et al, 2001) or smoking status (Travis et al, 1999).
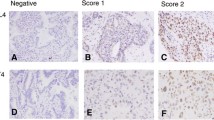
We observed a lower expression of hTERT in stage I NSCLC but no influence of telomerase levels of expression on survival rate. This point remains controversial (Hara et al, 2001; Toomey et al, 2001) although several authors strongly support the unfavourable prognostic value of a high telomerase expression in stage I NSCLC (Marchetti et al, 1999, 2002; Wang et al, 2002). Controversies regarding telomerase levels in tumours reside in the variety of technical approaches. Most previous data are based on telomerase activity measured by TRAP assay or quantification of hTERT mRNA by RT–PCR requiring samples containing at least 5000 viable tumour cells and obtained freshly in RNAse-free conditions. Sample contamination by telomerase negative normal epithelial or stromal cells might explain TRAP negative assay, whereas positive activated lymphocytes might contribute to a positive TRAP assay in the absence of telomerase activity in tumour cells (Cunningham et al, 1998; **narianos et al, 2000). A requirement of 70–80% of cancer cells seems reasonable in order to compare telomerase levels to external standards provided by cell lines (Marchetti et al, 2002). Furthermore, the exactitude of any measurement of telomerase activity is challenged by intratumoral heterogeneity of hTERT expression (Kumaki et al, 2001; Paradis et al, 2001). We and others have experienced successful hTERT in situ hybridisation approaches, which evaluate the level of transcription of hTERT (Kolquist et al, 1998; Soria et al, 2001; Wang et al, 2002). However, this technique remains time and labour-consuming. Therefore, the most promising tool for an in situ evaluation of telomerase expression is now represented by immunohistochemical detection of hTERT. To date, studies using noncommercially available antibodies have shown the nuclear expression of hTERT in tumour cells of various type, as well as in progenitor cells and activated lymphocytes and no expression in normal somatic cells. Among different commercially available antibodies against hTERT, only the monoclonal 44F12 antibody gave us both a unique and specific band on Western blotting and a clear-cut nuclear staining only in tumour component and activated lymphocytes. Furthermore, we found a high concordance between semiquantitative approaches of hTERT expression evaluated by immunohistochemistry and Western blotting on a same sample set.
A good correlation has been demonstrated between TRAP and hTERT immunohistochemical detection (Tahara et al, 1999; Kawakami et al, 2000; Kumaki et al, 2001, 2002) in colorectal tumours, liver tissues, lung cancer and mesothelioma. Our immunohistochemical approach in the setting of lung cancer was confronted to the TRAP assay as well as to hTERT in situ hybridisation and standard Western blotting. Similar profiles of RTA levels and hTERT staining scores were observed in lung tumours, higher levels being noted in SCLC and BC than in SCC and ADC. However in five cases where protein was detected by immunohistochemistry, telomerase activity was absent, and in 10 other cases, levels of telomerase expression evaluated by TRAP assay and immunohistochemistry were discordant. Such discrepancies might be explained by dilution of tumoral positive cells in the sample or by post-transcriptional and post-translational regulations of the protein quantitatively and qualitatively. As an example, the level of phosphorylation is able to control both telomerase activity (Li et al, 1997; Kang et al, 1999) and cytoplasmic vs nuclear localisation of hTERT (Kharbanda et al, 2000; Liu et al, 2001; Kyo and Inoue, 2002).
Interestingly, we reported for the first time a nucleolar localization of the catalytic subunit hTERT, preferentially located onto nucleolar structures in 45% of SCC and 42% of ADCs in contrast with its diffuse nuclear localization in all SCLC and 74% of BC. We have considered this pattern of staining as specific as it was observed in a number of TRAP and Western blot positive tumours. Indeed, compelling evidence has been provided that the assembly of hTERT subunit and hTERC RNA via box H/ACA motif takes place into the nucleolus favouring the hTERC maturation and the stabilization of the telomerase protein complex (Mitchell et al, 1999). Nucleolar localisation of hTERT seems also to occur independently of hTERC binding, suggesting that this phenomenon could correspond to a sequestration of hTERT away from its telomeric targets (Etheridge et al, 2002). In addition, subnuclear distribution of hTERT may vary according to cell cycle stage or DNA damage. Thus in normal cells, telomerase is released to the nucleoplasm during the S phase where it can add telomeric sequences to replicating chromosomes. In contrast, in response to ionising radiation, telomerase is excluded from the nucleoplasm and accumulated into the nucleolus in order to limit its accessibility to nontelomeric ends and to prevent its association to inappropriate substrates during the repair of NA breaks (Wong et al, 2002). Conversely in SV 40 transfected cells, oncogenic transformation triggers the releasing of hTERT into the nucleoplasmic compartment increasing the telomeric sequence synthesis (Wong et al, 2002). Since we report here a shorter survival in stage I NSCLC exhibiting a nucleolar pattern of staining, several hypotheses concerning the signification and the prognostic implication of nucleolar hTERT confinement may be proposed. The nucleolar localisation in some ADC and SCC is consistent with a regulated compartmentalised type of hTERT accumulation process where telomere elongation remains separated from DNA repair process during the S phase, thus protecting DNA from genetic instability and inopportune crisis (Wang et al, 2002). In contrast, concomitant nucleolar and nuclear distribution observed in aggressive tumours such as SCLC and BC is suggestive of a strong and aberrant increase of telomerase activation in fast-growing tumours that have acquired a large enough number of genetic lesions to escape senescence and apoptosis.
As telomerase inhibitors may be mainly effective after multiple cell divisions leading to cell death, they may have their greatest impact in combination with cytotoxic chemotherapy in advanced-stage disease and in high-grade tumours, such as BC and SCLC as well as in adjuvant therapy to surgery in early-stage disease. Although nucleolar localization in early NSCLC deserves specific attention, further studies need to be performed to improve our knowledge about telomerase regulation through its subnuclear distribution and cell cycle dependency in order to clarify the fundamental basis of the prognostic influence of hTERT nucleolar localisation.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Albanell J, Lonardo F, Rush V, Engelhardt M, Langenfeld J, Han W, Klimstra D, Venkatraman R, Moore MA, Dimitrovsky E (1997) High telomerase activity in primary lung cancers: association with increased cell proliferation rates and advanced pathologic stage. J Natl Cancer Inst 89: 1609–1615
Allsopp RC, Vaziri H, Patterson C, Golstein S, Younglai EV, Futcher AB, Greider CW, Harley CB (1992) Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci 89: 10114–10118
Ahrendt SA, Yang SC, Wu L, Westra WH, Jen J, Califano JA, Sidransky D (1997) Comparison of oncogene mutation detection and telomerase activity for the molecular staging of non small cell lung cancer. Clin Cancer Res 3: 1207–1214
Bacchetti S, Counter CM (1995) Telomeres and telomerase in human cancer. Int J Oncol 7: 423–432
Brambilla E, Moro D, Veale D, Brichon PY, Stoebner P, Paramelle B, Brambilla C (1992) Basal cell (basaloid) carcinoma of the lung. A new morphologic and phenotypic entity with separate prognostic significance. Hum Pathol 23: 993–1003
Brambilla E (1997) Basaloid carcinoma of the lung. In: Pathology of Lung Tumours, Corrin B (ed) pp 71–82. New York: Churchill-Livingstone
Carey LA, Hedican CA, Henderson GS, Umbricht CB, Dome JS, Varon D, Sukumar S (1998) Careful histological confirmation and microdissection reveal telomerase activity in otherwise telomerase negative breast cancers. Clin Cancer Res 4: 435–440
Counter CM, Avilion AA, Le Feuvre CE, Stewart NG, Greider CW, Harley CB, Bachetti S (1992) Telomerase shortening associated with chromosome instability is arrested in immortal cells with express telomerase activity. EMBO J 11: 1921–1929
Counter CM, Gupta J, Harley CB, Leber B, Bachetti S (1995) Telomerase activity in normal leucocytes and in haematology malignancies. Blood 85: 2315–2320
Cunningham VJ, Markham N, Shroyer LA (1998) Detection of telomerase expression in fine needle aspirations and fluids. Diagn Cytopathol 18: 431–436
Etheridge KT, Banik SSR, Armbruster BN, Zhu Y, Terns RM, Terns MP, Counter CM (2002) The nucleolar localization domain of the catalytic subunit of human telomerase. J Biol Chem 27: 24764–24770
Fujiwara M, Okayasu I, Takemura T, Tanaka I, Masuda R, Furuhata Y, Noji M, Oritsu M, Kato M, Oshimura M (2000) Telomerase activity significantly correlates with chromosome alterations, cell differentiation, and proliferation in lung adenocarcinoma. Mod Pathol 13: 723–729
Gomez-Roman JJ, Fontalba Romero A, Sanchez Castro L, Hernandez Nieto E, Fernandez-Luna JL, Val- Bernal JF (2000) Telomerase activity in pulmonary neuroendocrine tumours. Am J Surg Pathol 24: 417–421
Hara H, Yamashita, Shinada J, Yoshimura H, Kameya T (2001) Clinicopathologic significance of telomerase activity and hTERT mRNA expression in non small cell lung cancer. Lung Cancer 34: 219–226
Harley CB, Futcher AB, Greider CW (1990) Telomeres shortening during ageing of human fibroblasts. Nature 345: 458–460
Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC (1990) Telomere reduction in human colorectal carcinoma and with ageing. Nature 346: 866–868
Henderson EH (1995) Telomere DNA structure. In: Telomeres, Blackburn E, Greider C (eds) pp 11–34. New York: Cold Spring Harbor Laboratory Press
Hiyama E, Hiyama K, Yokoyama T, Matsura Y, Piatyszek MA, Shay JW (1995a) Correlating telomerase activity levels with neuroblastoma outcomes. Nat Med 1: 249–255
Hiyama E, Hokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Kodama T, Piatyszek MA, Shay JW, Matsura Y (1995b) Telomerase activity in gastric cancer. Cancer Res 55: 3258–3262
Hiyama K, Hiyama E, Ishioka S, Yamakido M, Inai K, Gazdar AF, Piatyszek MA, Shay JW (1995) Telomerase activity in small cell and non small cell lung cancer. J Natl Cancer Inst 87: 895–902
Holt SE, Shay JW (1999) Role of telomerase in cellular proliferation and cancer. J Cell Physiol 180: 10–18
Kang SS, Kwon T, Kwon DY, Do SI (1999) Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem 274: 13085–13090
Kawakami Y, Kitamoto M, Nakanishi T, Yasui W, Tahara E, Nakayama JI, Ishakawa F, Tahara H, Ide T, Kajiyama G (2000) Immunohistochemical detection of human telomerase reverse transcriptase in human liver tissues. Oncogene 19: 3888–3893
Kharbanda S, Kumar V, Dhar S, Pandey P, Chen C, Majumder P, Yuan ZM, Whang Y, Strauss W, Pandita TK, Weaver D, Kufe D (2000) Regulation of the hTERT telomerase catalytic subunit by the c-Abl tyrosine kinase. Curr Biol 10: 568–575
Kolquist KA, Ellisen LW, Counter CM, Meyerson M, Tan LK, Weinberg RA, Haber DA, Gerald WL (1998) Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet 19: 182–186
Kumaki F, Kawai T, Hiroi S, Shinomiya N, Oseki Y, Ferrans VJ, Torikata C (2001) Telomerase activity and expression of human telomerase RNA component and human telomerase reverse transcriptase in lung carcinomas. Hum Pathol 32: 188–195
Kumaki K, Kawai T, Churg A, Galateau- Sallé FB, Hasleton P, Henderson D, Roggli V, Travis WD, Cagle PT, Ferrans VJ (2002) Expression of telomerase reverse transcriptase (TERT) in malignant mesotheliomas. Am J Surg Pathol 26: 365–370
Kyo S, Inoue M (2002) Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy? Oncogene 21: 688–697
Langford LA, Piatyszek MA, Xu R, Schold SC, Shay JW (1995) Telomerase activity in human brain tumours. Lancet 346: 1267–1268
Li H, Zhao L, Yang Z, Funder JW, Liu JP (1997) Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J Biol Chem 272: 16729–16732
Liu K, Hodes RJ, Weng NP (2001) Cutting edge: Telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J Immunol 166: 4826–4830
Marchetti A, Bertacca G, Buttita F, Chella A, Quattrocolo G, Angeletti CA, Belivacqua G (1999) Telomerase activity as a prognostic indicator in stage I non small cell lung cancer. Clin Cancer Res 5: 2077–2081
Marchetti A, Pellegrini C, Buttitta F, Falleni M, Romagnoli S, Felicioni L, Barassi F, Salvatore S, Chella A, Angelletti CA, Roncalli M, Coggi G, Bosari S (2002) Prediction of survival in stage I lung carcinoma patients by telomerase function evaluation. Lab Invest 82: 729–736
Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddie SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bachetti S, Haber DA, Weinberg RA (1997) hETS2 the putative human telomerase catalytic subunit gene, is up-regulated in tumour cells and during immortalization. Cell 90: 785–795
Mitchell JR, Wood E, Collins K (1999) A telomerase component is detective in the human disease dyskeratosis congenita. Nature (London) 402: 551–555
Nakamura TM, Cech TR (1998) Reversing time: origin of telomerase. Cell 92: 587–590
Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Linger J, Harley CB, Cech TR (1997) Telomerase catalytic subunit homologs from fission yeast and human. Science (Wash DC) 277: 955–959
Nakanishi K, Kawai T, Kumaki F, Hirot S, Mukai M, Ikeda E (2002) Expression of human telomerase RNA component and telomerase reverse transcriptase mRNA in atypical adenomatous hyperplasia of the lung. Hum Pathol 33: 697–702
Paradis V, Bieche I, Dargere D, Bonvoust F, Ferlicot S, Olivi M, Ben Lagha N, Blanchet P, Benoit G, Vidaud M, Bedossa P (2001) HTERT expression in sporadic renal cell carcinomas. J Pathol 195: 209–217
Soria JC, Moon C, Wang L, Hittelman WN, Jang SJ, Sun SY, Lee JJ, Liu D, Kurie JM, Morice RC, Lee SJ, Hong WK, Mao L (2001) Effects of N-(4-Hydroxyphenyl) retinamide on hTERT expression in the bronchial epithelium of smokers. J Natl Cancer Inst 93: 1257–1263
Tahara H, Yasui W, Tahara E, Fujimoto J, Ito K, Tamai K, Nakayama JI, Ishakawa F, Tahara E, Ide T (1999) Immunohistochemical detection of human telomerase catalytic component hTERT in human colorectal tumour and non tumour tissue sections. Oncogene 18: 1561–1567
Toomey D, Smyth G, Condron C, Kay E, Conroy R, Foley D, Hong C, Hogan B, Toner S, McCormick P, Broe P, Kelly C, Bouchier-Hayes D (2001) Immune function, telomerase and angiogenesis in patients with primary, operable non small cell lung carcinoma: tumour size and lymph node status remain the most important prognostic features. Cancer 92: 2648–2657
Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E (1999) Histological Ty** of Lung and Pleural Tumours. World Health Organization. International Histological Classification of Tumours, 3rd edn. Berlin: Springer-Verlag
Wang L, Soria JC, Kemp BL, Liu DD, Mao L Khuri FR (2002) hTERT expression is a prognostic factor of survival in patients with stage I non small cell lung cancer. Clin Cancer Res 8: 2883–2889
Wick M, Zubov D, Hagen G (1999) Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT). Gene 232: 97–106
Wong JMY, Kusdra L, Collins K (2002) Sub nuclear shuttling of human telomerase induced by transformation and DNA damage. Nat Cell Biol 4: 731–736
**narianos G, Scott FM, Liloglou T, Prime W, Callaghan J, Gosney JR, Field JK (1999) Telomerase activity in non small cell lung carcinomas correlates with smoking status. Int J Oncol 15: 961–965
**narianos G, Scott FM, Liloglou T, Prime W, Callaghan J, Gosney JR, Field JK (2000) Evaluation of telomerase activity in bronchial lavage as a potential diagnostic marker for malignant lung disease. Lung Cancer 28: 37–42
Yashima K, Litzky LA, Kaiser L, Rogers T, Lam I, Wistuba II, Milchgrub S, Srivastava S, Piatyszek MA, Shay JW, Gazdar AF (1997) Telomerase expression in respiratory epithelium during the multistage pathogenesis of lung carcinomas. Cancer Res 57: 2373–2377
Acknowledgements
Work in INSERM U 578 is funded by La Ligue Contre Le Cancer, l’Association de Recherche sur le Cancer (ARC) and le Projet Hospitalier de Recherche Clinique (PHRC). Work in LS laboratory is funded by CEC SUS GENINRADCAR FIGH 1999-00002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Lantuejoul, S., Soria, J., Moro-Sibilot, D. et al. Differential expression of telomerase reverse transcriptase (hTERT) in lung tumours. Br J Cancer 90, 1222–1229 (2004). https://doi.org/10.1038/sj.bjc.6601643
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601643
- Springer Nature Limited
Keywords
This article is cited by
-
Immunohistochemical investigation of prognostic biomarkers in resected colorectal liver metastases: a systematic review and meta-analysis
Cancer Cell International (2018)
-
A phase I/randomized phase II study of GM.CD40L vaccine in combination with CCL21 in patients with advanced lung adenocarcinoma
Cancer Immunology, Immunotherapy (2018)
-
The associations of TERT-CLPTM1L variants and TERT mRNA expression with the prognosis of early stage non-small cell lung cancer
Cancer Gene Therapy (2017)
-
Predictive value of telomerase reverse transcriptase expression in patients with high risk superficial bladder cancer treated with adjuvant BCG immunotherapy
Journal of Cancer Research and Clinical Oncology (2009)
-
Neuro-endocrine tumours of the lung. A review of relevant pathological and molecular data
Virchows Archiv (2007)