Abstract
The research employed network toxicology and molecular docking techniques to systematically examine the potential carcinogenic effects and mechanisms of aspartame (l-α-aspartyl-l-phenylalanine methyl ester). Aspartame, a commonly used synthetic sweetener, is widely applied in foods and beverages globally. In recent years, its safety issues, particularly the potential carcinogenic risk, have garnered widespread attention. The study first constructed an interaction network map of aspartame with gastric cancer targets using network toxicology methods and identified key targets and pathways. Preliminary validation was conducted through microarray data analysis and survival analysis, and molecular docking techniques were employed to further examine the binding affinity and modes of action of aspartame with key proteins. The findings suggest that aspartame has the potential to impact various cancer-related proteins, potentially raising the likelihood of cellular carcinogenesis by interfering with biomolecular function. Furthermore, the study found that the action patterns and pathways of aspartame-related targets are like the mechanisms of known carcinogenic pathways, further supporting the scientific hypothesis of its potential carcinogenicity. However, given the complexity of the in vivo environment, we also emphasize the necessity of validating these molecular-level findings in actual biological systems. The study introduces a fresh scientific method for evaluating the safety of food enhancers and provides a theoretical foundation for sha** public health regulations.
Similar content being viewed by others
Introduction
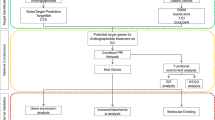
Aspartame, also called l-α-aspartyl-l-phenylalanine methyl ester, is an artificial sweetener with few calories. Since its discovery in 1965, it has been approved in over ninety countries worldwide for use in thousands of food products and beverages. These products include sugar-free sodas, diet drinks, low-sugar or sugar-free desserts, chewing gum, flavor packets, certain medications, and energy drinks1. Due to its sweetness being around two hundred times greater than sucrose, only a small quantity is needed to reach the desired level of sweetness in food items, which is why it is favored by diabetics and individuals aiming to shed pounds2. However, there remains controversy among the public and the scientific community regarding the health risks associated with long-term consumption, particularly its carcinogenicity. This controversy persists even after the World Health Organization (WHO) classified aspartame as a carcinogenic substance3. The U.S. Food and Drug Administration (FDA) continues to regard aspartame as safe within the recommended intake limits, setting the Acceptable Daily Intake (ADI) at 40 mg per kilogram of body weight4. Part of the reason for this controversy is that early toxicological studies were based on high-dose animal experiments and could not be directly inferred to low-dose human exposure5. Additionally, inconsistencies in the results of different studies, as well as limited understanding of aspartame's metabolic pathways and the mechanisms of action of its metabolites, add to the complexity of assessing its safety6. Furthermore, additional research indicates that aspartame may pose a risk factor for diseases such as cerebrovascular disorders7, depression8, and autism9. Currently, there is still controversy regarding the safety of aspartame in epidemiological research findings, with some studies suggesting it as a risk factor for tumors while others reach the opposite conclusion10,11. With the advancement of toxicological research methods, especially the emergence of network toxicology, researchers have begun to analyze from a systems biology perspective how chemicals affect the stability of biological systems. Network toxicology integrates bioinformatics, systems biology, and toxicology to systematically study the interactions between chemicals and biological systems at the molecular level, and how these interactions lead to toxic effectsS1)43.
We noticed an increase in MMP9 and a decrease in CASP3 when comparing the gene expression patterns of healthy stomach tissue with those of stomach cancer tissue. This may suggest that in gastric cancer, there is an enhanced degradation of the extracellular matrix and an increased invasive capability of the tumor (indicated by the upregulation of MMP9), while the mechanisms of cell apoptosis are simultaneously inhibited (indicated by the downregulation of CASP3). The expression levels of MMP9 and CASP3 are significantly correlated with the overall survival time of patients with gastric cancer. Specifically, high expression of MMP9 may be associated with a poorer prognosis, while low expression of CASP3 may further exacerbate the malignancy of the tumor.
Matrix metalloproteinase 9 (MMP9) is a key enzyme involved in degrading the extracellular matrix (ECM), responsible for breaking down various components within the ECM, including collagen and fibronectin. During the development of gastric cancer, the increased activity of MMP9 is crucial for tumor cells to penetrate the basement membrane and ECM, facilitating tumor cell invasion into neighboring tissues and their dissemination to new sites through the bloodstream or lymphatic system44. By degrading ECM, MMP9 not only alters the physical and chemical environment surrounding tumor cells, thereby promoting tumor cell proliferation and survival, but also influences the tumor immune microenvironment. It indirectly regulates the infiltration and function of immune cells by adjusting the composition and structure of ECM, thereby impacting the overall immune response45. Furthermore, through its degradative action on ECM, MMP9 paves the way for the formation of new blood vessels within tumors, which is crucial for tumor blood supply, oxygen delivery, as well as further growth and dissemination46. The expression and activity of MMP9 are regulated by various tumor-related factors, including TGF-β, EGF, and FGF, which participate in the regulation of multiple signaling pathways involved in tumor initiation and progression47. In some cases, MMP9 is also associated with the resistance of tumor cells to chemotherapy drugs, possibly by altering the composition and density of the ECM, thereby affecting the distribution and efficacy of drugs48. It is worth noting that MMP9 does not act in isolation; there is a complex mutual regulatory relationship between MMP9 and other members of the MMP family and their inhibitors (TIMPs).
Caspase-3 (CASP3) plays a core role in the process of cell apoptosis, which is a precisely regulated mechanism of cellular self-destruction crucial for maintaining tissue health and physiological balance. In the progression of cancer, especially in gastric cancer development, the apoptosis pathway is often disrupted, a common feature of various malignant tumors49. Inhibition of CASP3 activity may lead to increased resistance of gastric cancer cells to certain chemotherapy drugs, thereby reducing the effectiveness of treatment50. Moreover, the complex interaction between tumor cells and their microenvironment, including surrounding non-tumor cells, extracellular matrix, and signaling molecules, may be affected by abnormal CASP3 function. This abnormality may not only disrupt the invasiveness and metastatic potential of tumors but also impact the overall growth dynamics of tumors. Although closely associated with cell death, CASP3 has also been shown to promote the migration and invasion of tumor cells through non-traditional pathways other than apoptosis under specific conditions51,52. Additionally, in certain circumstances, the expression level of CASP3 is closely related to the prognosis of gastric cancer patients, with reduced expression often associated with higher tumor grades, poor prognosis, and lower survival rates in patients53.
We also conducted molecular docking studies, a computational method for simulating the binding of drug molecules to their target proteins. A decrease in docking energy indicates a higher level of interaction between the molecules, resulting in a more secure binding. Our results indicate that the binding energies of aspartame to these key proteins range from − 5.538 to − 7.75 kcal/mol, with the highest binding energy to MMP9 (− 7.75 kcal/mol), suggesting a potentially strong interaction between aspartame and MMP9.This is consistent with the findings of the upregulated expression of the MMP9 gene.
Conclusions
The findings suggest that aspartame has the potential to impact various cancer-related proteins, potentially raising the likelihood of cellular carcinogenesis by interfering with biomolecular function. Furthermore, the study found that the action patterns and pathways of aspartame-related targets are like the mechanisms of known carcinogenic pathways, further supporting the scientific hypothesis of its potential carcinogenicity.
In summary, our study underscores the importance of continued investigation into the relationship between artificial sweeteners and cancer, which may impact the diagnostic and therapeutic strategies for gastric cancer. Future research should focus on revealing how aspartame contributes to the development of gastric cancer by affecting cellular signaling pathways, as well as how this knowledge can be utilized to develop novel treatment modalities.
While our research offers fresh perspectives on the possible connection between aspartame and stomach cancer, there are constraints to consider. Firstly, our research is based on observational biomarker expression patterns, which limits our interpretation of causality. Secondly, while microarray data is a powerful tool, its results need to be validated through independent methods such as quantitative PCR, Western Blotting, or immunohistochemistry. Additionally, our results need to be confirmed in larger, separate groups and supported by epidemiological research to determine the connection between consuming aspartame and the likelihood of develo** gastric cancer.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Schorb, S. et al. Assessment of aspartame (E951) occurrence in selected foods and beverages on the German market 2000–2022. Foods 12, 2156 (2023).
Czarnecka, K. et al. Aspartame—true or false? Narrative review of safety analysis of general use in products. Nutrients 13, 1957 (2021).
Naddaf, M. Aspartame is a possible carcinogen: The science behind the decision. Nature https://doi.org/10.1038/d41586-023-02306-0 (2023).
Harris, E. Experts disagree about aspartame’s “possibly carcinogenic” status. JAMA 330, 585 (2023).
Schernhammer, E. S. et al. Consumption of artificial sweetener- and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am. J. Clin. Nutr. 96, 1419–1428 (2012).
Landrigan, P. J. & Straif, K. Aspartame and cancer—new evidence for causation. Environ. Health 20, 42 (2021).
Girigosavi, K. B., Etta, I., Kambham, S. & Panjiyar, B. K. Sweet surprises: An in-depth systematic review of artificial sweeteners and their association with cerebrovascular accidents. Curr. Nutr. Rep. https://doi.org/10.1007/s13668-024-00537-9 (2024).
Ma, H., Deng, J., Liu, J., **, X. & Yang, J. Daytime aspartame intake results in larger influences on body weight, serum corticosterone level, serum/cerebral cytokines levels and depressive-like behaviors in mice than nighttime intake. NeuroToxicology 102, 37–47 (2024).
Fowler, S. P. et al. Daily early-life exposures to diet soda and aspartame are associated with autism in males: A case-control study. Nutrients 15, 3772 (2023).
Goodman, J. E., Boon, D. N. & Jack, M. M. Perspectives on recent reviews of aspartame cancer epidemiology. Glob. Epidemiol. 6, 100117 (2023).
Pavanello, S., Moretto, A., La Vecchia, C. & Alicandro, G. Non-sugar sweeteners and cancer: Toxicological and epidemiological evidence. Regul. Toxicol. Pharmacol. 139, 105369 (2023).
Liu, C., Fan, H., Li, Y. & **ao, X. Research advances on hepatotoxicity of herbal medicines in China. BioMed Res. Int. 2016, 1–14 (2016).
Zdrazil, B. et al. The ChEMBL Database in 2023: A drug discovery platform spanning multiple bioactivity data types and time periods. Nucleic Acids Res. 52, D1180–D1192 (2024).
Szklarczyk, D. et al. STITCH 5: Augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 44, D380–D384 (2016).
Daina, A., Michielin, O. & Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47, W357–W364 (2019).
Keiser, M. J. et al. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 25, 197–206 (2007).
Wang, X. et al. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 45, W356–W360 (2017).
UniProt Consortium. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 51, D523–D531 (2023).
Stelzer, G. et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 54, 1.30.1-1.30.33 (2016).
McKusick, V. A. Mendelian inheritance in man and its online version. OMIM. Am. J. Hum. Genet. 80, 588–604 (2007).
Piñero, J., Saüch, J., Sanz, F. & Furlong, L. I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 19, 2960–2967 (2021).
Szklarczyk, D. et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Otasek, D., Morris, J. H., Bouças, J., Pico, A. R. & Demchak, B. Cytoscape automation: Empowering workflow-based network analysis. Genome Biol. 20, 185 (2019).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Sherman, B. T. et al. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221 (2022).
**, Z., Sato, Y., Kawashima, M. & Kanehisa, M. KEGG tools for classification and analysis of viral proteins. Protein Sci. 32, e4820 (2023).
Tang, D. et al. SRplot: A free online platform for data visualization and graphing. PLoS ONE 18, e0294236 (2023).
Dai, W., Li, Q., Liu, B.-Y., Li, Y.-X. & Li, Y.-Y. Differential networking meta-analysis of gastric cancer across Asian and American racial groups. BMC Syst. Biol. 12, 51 (2018).
Zhang, X. et al. Overexpression of E2F mRNAs associated with gastric cancer progression identified by the transcription factor and miRNA co-regulatory network analysis. PLoS ONE 10, e0116979 (2015).
Edgar, R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Győrffy, B. Transcriptome-level discovery of survival-associated biomarkers and therapy targets in non-small-cell lung cancer. Br. J. Pharmacol. 181, 362–374 (2024).
Morris, G. M., Huey, R. & Olson, A. J. Using AutoDock for ligand-receptor docking. Curr. Protoc. Bioinform. 24, 8.14 (2008).
Kim, S. et al. PubChem 2023 update. Nucleic Acids Res. 51, D1373–D1380 (2023).
Berman, H., Henrick, K. & Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 10, 980–980 (2003).
Hers, I., Vincent, E. E. & Tavaré, J. M. Akt signalling in health and disease. Cell. Signal. 23, 1515–1527 (2011).
LaRock, C. N. et al. IL-1β is an innate immune sensor of microbial proteolysis. Sci. Immunol. 1, eaah3539 (2016).
Wang, Y., Cao, H., Chen, J. & McNiven, M. A. A direct interaction between the large GTPase dynamin-2 and FAK regulates focal adhesion dynamics in response to active Src. Mol. Biol. Cell 22, 1529–1538 (2011).
Ghosh, P. et al. A Gαi–GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol. Biol. Cell 21, 2338–2354 (2010).
Simoneschi, D. et al. CRL4AMBRA1 is a master regulator of D-type cyclins. Nature 592, 789–793 (2021).
Sun, M., Song, L., Li, Y., Zhou, T. & Jope, R. S. Identification of an antiapoptotic protein complex at death receptors. Cell Death Differ. 15, 1887–1900 (2008).
Greten, F. The IKK/NF-κB activation pathway—a target for prevention and treatment of cancer. Cancer Lett. 206, 193–199 (2004).
Fu, C.-K. et al. Association of Matrix Metallopeptidase-2 genotypes with risk of gastric cancer in Taiwan. Anticancer Res. 42, 1749–1755 (2022).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Lochter, A., Sternlicht, M. D., Werb, Z. & Bissell, M. J. The significance of matrix metalloproteinases during early stages of tumor progressiona. Ann. N. Y. Acad. Sci. 857, 180–193 (1998).
Liu, B. et al. CircUBAP2 promotes MMP9-mediated oncogenic effect via sponging miR-194-3p in hepatocellular carcinoma. Front. Cell Dev. Biol. 9, 675043 (2021).
Oh, E., Hong, J. & Yun, C.-O. Regulatory T cells induce metastasis by increasing Tgf-β and enhancing the epithelial-mesenchymal transition. Cells 8, 1387 (2019).
Son, H.-K., Kim, D., Lim, Y., Kim, J. & Park, I. A novel TGF-β receptor II mutation (I227T/N236D) promotes aggressive phenotype of oral squamous cell carcinoma via enhanced EGFR signaling. BMC Cancer 20, 1163 (2020).
Jiguet-Jiglaire, C. et al. Plasmatic MMP9 released from tumor-infiltrating neutrophils is predictive for bevacizumab efficacy in glioblastoma patients: An AVAglio ancillary study. Acta Neuropathol. Commun. 10, 1 (2022).
Li, B. et al. Novel pathways of HIV latency reactivation revealed by integrated analysis of transcriptome and target profile of bryostatin. Sci. Rep. 10, 3511 (2020).
Lin, Y.-F. et al. Targeting the XIAP/caspase-7 complex selectively kills caspase-3–deficient malignancies. J. Clin. Investig. 123, 3861–3875 (2013).
Fabbri, M. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA 305, 59 (2011).
Bhatia, V., Mula, R. V. R. & Falzon, M. Parathyroid hormone-related protein regulates integrin α6 and β4 levels via transcriptional and post-translational pathways. Exp. Cell Res. 319, 1419–1430 (2013).
Wang, Z. et al. Prognostic significance of mRNA expression of CASPs in gastric cancer. Oncol. Lett. 18, 4535–4554 (2019).
Author information
Authors and Affiliations
Contributions
Both Chen Dandan and Hou **anbing participated in the whole process of paper writing, including research design, data mining, data analysis, and article writing. All authors approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, D., Hou, X. Aspartame carcinogenic potential revealed through network toxicology and molecular docking insights. Sci Rep 14, 11492 (2024). https://doi.org/10.1038/s41598-024-62461-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62461-w
- Springer Nature Limited