Abstract
Cervicogenic headache is an often observed secondary headache in clinical settings, with patients who endure prolonged and persistent pain being particularly susceptible to mood changes. Currently, the Mulligan is one of the effective methods for CEH. However, there is a lack of evaluation about the strength and frequency of headaches, as well as the assessment of pain-induced emotions, in individuals with CEH using this particular procedure. Herein, we aimed to evaluate the effectiveness of the Mulligan maneuver from a multidimensional perspective of pain intensity and mood. A total of forty patients diagnosed with CEH who satisfied the specified inclusion criteria were recruited and allocated randomly into two groups: the control group and the treatment group, with each group consisting of twenty cases. The control group received health education, while the treatment group received the Mulligan maneuver once daily over a course of 10 treatment sessions.The clinical outcome of patients with CEH in two groups was assessed using the Visual Analog Scale (VAS), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD). Resting-state functional magnetic resonance imaging was employed to examine variations in brain function activities between the two CEH groups. Brain regions showing differences were identified as regions of interest and subsequently correlated with clinical behavioral measures using Pearson’s correlation analysis. The differences in VAS, HAMA and HAMD between the two groups of CEH patients were also statistically significant. The brain regions that showed differences in the ReHo scores between the two groups of CEH patients included the left cerebellum, the frontal gyrus, and the middle temporal gyrus. There was a positive correlation between the left frontal gyrus and VAS, HAMA and HAMD. The left middle temporal gyrus had a negative correlation with VAS, HAMA, and HAMD and the left cerebellum had a positive correlation with VAS correlation. The Mulligan maneuver may improve pain levels and have a moderating effect on pain-related negative emotions by regulating the function of relevant brain regions in CEH patients.
Similar content being viewed by others
Introduction
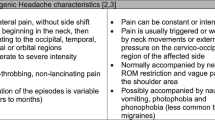
Cervicogenic headache (CEH) is one of the most common secondary headaches in clinical practice and is caused by bony, disc, or soft tissue disorders of the cervical spine1. CEH is a form of entrapment pain and explained its pathogenesis with the congregation theory. According to this theory, lesions in the structures innervated by the high cervical nerves (the greater occipital, lesser occipital, and greater auricular nerves belong to the second and third cervical nerves) cause afferent injurious sensory messages from the high cervical nerves that are connected2. The prevalence of cervicogenic headache was estimated at 1%, 2.5%3 or 4 0.1%4 of the total population and up to 17.5% in patients with severe headaches3. The Prevalence is up to 53% in patients with post-whiplash headache5. The clinical diagnostic criteria include unilateral headache with evidence of cervical involvement by provocation of pain by neck motion or pressure on the neck; concomitant pain in the neck, shoulder and arm; and reduced neck range of motion, with or without other features6. Approximately 54% of CEH patients can develop chronic intractable pain. This can lead to anxiety or depression, seriously affecting the daily activities of patientsOutcome The primary outcomes were assessed by an assessor blinded to group allocation and intervention in the department at each time point, such as at baseline and after ten sessions of treatment. The secondary outcomes were assessed by a technician trained in magnetic resonance operation, but blinded to group allocation and intervention after patients had completed ten sessions of treatment. CEH pain: A 10-point visual analogue scale (VAS) was used to measure the intensity of CEH pain14. Participants were asked to rate the intensity of pain on a 10 cm scale, where0 being ‘no pain’ and 10 being ‘maximum pain that is unbearable’. This is a valid and reliable (ICC = 0.60–0.77) instrument for measuring CEH pain intensity and was administered at baseline and after ten sessions of treatment. CEH emotion: Hamilton Anxiety Scale (HAMA) scale was used to assess anxiety symptoms15. A scale of psychological and physical anxiety was completed by participants, ranging from 0 to 4, indicating no symptoms to extremely severe symptoms. Hamilton Depression (HAMD) scale was used to assess the depression symptoms15. Symptoms were scored on a five-point scale, where 0 to 4 ranged from no symptoms to extremely severe symptoms and included 7 subscales: anxiety/somatisation, weight, cognitive impairment, diurnal variation, blockage, sleep disturbance, and hopelessness.The test–retest reliability was between 0.65 and 0.91 ,and the construct validity was good. The local brain functional activity: Regional Homogeneity (ReHo) is a measure of the local coherence of spontaneous brain activity and is sensitive to the following abnormal local functional connectivity of brain regions16. ReHo combined with clinical variables has been used to study pain and psychiatric disorders. This study was a randomized controlled trial where in two groups were established based on the pre-experiment outcomes. The sample size for this study was calculated based on the primary outcome of visual analogue scale. According to the literature17, the sample size estimation formula for comparing the two sample rates is as follows: SD—Standard deviation = pre-experiment outcomes; Za/2 = Z0.05/2 = Z0.025 = 1.96 (From Z table) at type I error of 5%; Zβ = Z0.20 = 0.842 (From Z table) at 80% power, d = effect size = difference between mean values. The allocation of participants into the test and control groups followed a 1:1 ratio. Each group initially consisted of 16 cases, and the sample size was increased by 20% to account for potential factors including loss of visits and attrition. Consequently, a minimum of 20 cases per group was required for the study subjects. Data were statistically analyzed using the SPSS26 software package (SPSS Inc. USA). Measurement data was described using \(\overline{x }\) ± s and count data were described using constitutive ratios and frequencies. Independent samples t-test and paired rank sum test (Z-test) were used to compare the differences in indicators between the two groups of CEH patients. The RESTplusv1.24 software package was used for statistical analysis of the data. The smReHo plots of CEH patients in the two groups were subjected to paired samples t-test, corrected for multiple comparisons by the GRF method (P < 0.01 for voxel level, P < 0.05 for clusters). The ReHo values of different brain regions were extracted and analyzed by Pearson correlation analysis with clinical behavior indices (VAS, HAMA, HAMD), and the differences were considered statistically significant at P < 0.05.Primary outcomes
Secondary outcome
Sample size calculation
Statistical analysis
Results
Group characteristics
The clinical experiment involved a cohort of 40 patients, with 20 assigned to the Mulligan maneuver therapy group and the remaining 20 assigned to the health promotion intervention group. There were no statistically significant differences in the general age, sex, education level, disease duration, VAS score, HAMA score, and HAMD score of the patients in the two groups before the trial (P > 0.05) (Table 1).
CEH pain results
According to the test of normality, the scores of the VAS scale of the two groups were in accordance with a normal distribution. After ten sessions of treatment, CEH VAS score 1.16(95% CI 0.49 to 1.82) improve (p = 0.00) in the Mulligan maneuver therapy group more than in the health promotion intervention group (Table 2).
CEH emotion results
After testing for normality, the Hamilton Anxiety Scale (HAMA) and the Hamilton Depression (HAMD) scale scores of the two groups were found to follow a normal distribution. After ten sessions of treatment, HAMA score 1.08 (95% CI 0.08816 to 2.072) improve (p = 0.03) and HAMD score 1.62 (95% CI 0.3606 to 2.879) improve (p = 0.01) in the Mulligan maneuver therapy group more than in the health promotion intervention group (Table 2).
The local brain functional activity
Moreover, there were differences in the changes in ReHo scores of the left cerebellum, left middle frontal gyrus, and left middle temporal gyrus between the two groups (P < 0.01) (Fig. 3) (Table 3). Furthermore, we observed a positive correlation between the ReHo values of left frontal gyrus and the VAS (R2 = 0.48, P = 0.00), HAMA (R2 = 0.28, P = 0.03), and HAMD (R2 = 0.26, P = 0.03) scores in the treatment group (Fig. 4). Conversely, in the treatment group, we observed a negative correlation between the ReHo values of left temporal middle gyrus and the VAS (R2 = 0.44, P = 0.00), HAMA (R2 = 0.43, P = 0.00), and HAMD (R2 = 0.25, P = 0.03) score(Fig. 5). The ReHo value of the cerebellum in the treatment group was positively correlated with the VAS score (R2 = 0.27, P = 0.03). However, no significant correlations were found between HAMA (R2 = 0.14, P = 0.13) and the HAMD scores (R2 = 0.04, P = 0.4) (Fig. 6).
Discussion
Chronic persistent pain can lead to cognitive abnormalities in pain, subsequently causing anxiety or depression. Recent research shows that CEH patients suffer from the physical discomfort of pain, cognitive dysfunction, and psychological dysphoria18. A previous study has shown that CEH patients suffer from physical pain, cognitive dysfunction, and psychological distress. Our research supports this view. In addition to the higher VAS score, the HAMA and HAMD score of the CEH patients was also higher than that of the normal patients. This is an indication of depression or anxiety. Previous studies have demonstrated the effectiveness of manipulative therapy in terms of headache index, trigger point pressure pain, neck function assessment index, and range of motion in CEH19, and the evaluation of pain-induced emotions is less involved. The results showed: the VAS score, the HAMA and HAMD score scores decreased after the Mulligan maneuver treatment and were better than in the control group. This suggests that the Mulligan manoeuvre has a therapeutic effect on the intensity of pain, and also has some alleviating effect on pain-induced anxiety or depression.
Moreover, an additional study has shown that CEH patients have synergistic increases or decreases in neuronal activity in localized brain tissue, suggesting that CEH involves pathological changes in multiple brain regions associated with regulating affective cognition20. Our study is an innovative use of fMRI technology to explore the functional areas of the brain where the Mulligan maneuver has therapeutic effects, thus providing a scientific basis for the Mulligan maneuver treatment of CEH. This method has a certain uniqueness.
Herein, we found statistically significant changes in the function of frontal, middle gyrus, and middle temporal gyrus brain regions in CEH patients using the Mulligan maneuver. Furthermore, we identified correlations between these changes and clinical behavioral indicators. The middle frontal gyrus is a part of the frontal lobe that is thought to play a crucial role in regulating affective cognition, including memory and executive function. It is important to reduce pain sensitivity through affective cognitive modulation21. In conjunction with the results of this project, we believe that CEH patients exposed to repeated painful stimuli who are subjected to recurrent noxious stimuli experience specific aberrations in the perception and modulation of pain-related data. Several below-threshold pain tends to be amplified and processed. This is related to the fact that long-term chronic pain tends to cause damage to the frontal gyrus and emotional cognitive decline in patients with CEH. The Mulligan maneuver can somewhat improve the functional changes in the frontal gyrus and other brain regions and alleviate pain sensitivity due to cognitive decline.
The middle temporal gyrus is also a target of the Mulligan maneuver. Several studies have reported that the middle temporal gyrus is associated with emotional and sensory processing, including depression, anxiety, and pain ratings. Furthermore, it has been observed that enduring unpleasant emotional experiences resulting from pain can potentially create alterations in brain functionality. Herein, ReHo scores in the middle temporal gyrus were reduced and negatively correlated with VAS, HAMA, and HAMD, possibly due to the Mulligan maneuver’s protective inhibitory effect on the middle temporal gyrus. MEDINA22 study has found that cerebral blood flow in the middle temporal gyrus was reduced after treating CEH patients and Mohamadi23 study also shows that Manipulation affects central sensitization which can change brain metabolic map. This is consistent with our findings. So we hypothesise that by using the Mulligan technique, sensory inputs can be sent to the CNS. Perhaps these additional sensory inputs play a role in these inhibitory effects and we believe that the Mulligan maneuver can regulate the pain level and play a significant role in pain-induced emotions.
Additionally, we found that the manipulation had a benign moderating effect on changes in cerebellar function and a positive correlation between this change and VAS scores. Previous studies have focused on the cerebral cortex at the expense of cerebellar function. In a rat model of neuropathic pain, cerebellar activity was positively correlated with the development of neuropathic pain and prognosis24. The cerebellum plays an important role in processing pain sensations, as signal inputs from the A-fibre and C-fibre nociceptors reach the Purkinje cells of cerebellum25. The cerebellum is closely connected to the brain’s limbic system, amygdala, hippocampus, anterior cingulate gyrus, and frontal and temporal lobes. According to our findings, we believe that the Mulligan maneuver adjusts the neural circuits between the cerebellum and the brain by modulating the functional areas of cerebellum, thereby activating the corresponding functional areas of brain and ultimately realizing the intervention of pain emotion.
Limitations and suggestions
Nevertheless, it is important to acknowledge the constraints of this investigation, namely the relatively small sample size and the utilization of imaging data solely for the purpose of examining alterations in regional brain functionality among CEH patients. Consequently, further investigations need to be conducted to increase the sample size and explore the underlying mechanisms pertaining to the network connectivity of local cerebral activity.
Conclusions
Herein, we found that the Mulligan maneuver can improve pain levels and regulate pain-induced negative emotions by modulating the function of relevant brain regions in CEH patients.
Data availability
The data sets that were used and/or analysed in the current study are available from the corresponding author upon reasonable request.
References
Olesen, D., Bes, A. & Kunkel, R. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 38(1), 1–211 (2018).
Bogduk, N. Cervicogenic headache: Anatomic basis and pathophysiologic mechanisms. Curr. Pain Headache Rep. 5(4), 382–386 (2001).
Evers, S. Comparison of cervicogenic headache with migraine. Cephalalgia 28(Suppl 1), 16–17 (2008).
Sjaastad, O. Cervicogenic headache: Comparison with migraine without aura; Vågå study. Cephalalgia 28(Suppl 1), 18–20 (2008).
Lord, S. M. et al. Third occipital nerve headache: A prevalence study. J. Neurol. Neurosurg. Psychiatry 57(10), 1187–1190 (1994).
Bogduk, N. & Govind, J. Cervicogenic headache: An assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 8(10), 959–968 (2009).
**ao, H. et al. The Chinese association for the study of pain (CASP): Expert consensus on the cervicogenic headache. Pain Res. Manag. 2019, 9617280 (2019).
Satpute, K., Bedekar, N. & Hall, T. Effectiveness of Mulligan manual therapy over exercise on headache frequency, intensity and disability for patients with migraine, tension-type headache and cervicogenic headache - A protocol of a pragmatic randomized controlled trial. BMC Musculoskelet. Disord. 22(1), 243 (2021).
**, X. et al. The efficiency and safety of manual therapy for cervicogenic cephalic syndrome (CCS): A systematic review and meta-analysis. Medicine (Baltimore) 100(8), e24939 (2021).
Wen, Y. et al. A spinal manipulative therapy altered brain activity in patients with lumbar disc herniation: A resting-state functional magnetic resonance imaging study. Front. Neurosci. 16, 974792 (2022).
Chen, X. M. et al. Traditional Chinese manual therapy (Tuina) reshape the function of default mode network in patients with lumbar disc herniation. Front. Neurosci. 17, 1125677 (2023).
Núñez-Cabaleiro, P. & Leirós-Rodríguez, R. Effectiveness of manual therapy in the treatment of cervicogenic headache: A systematic review. Headache 62(3), 271–283 (2022).
David, J. P., Helbig, T. & Witte, H. SenGlove-a modular wearable device to measure kinematic parameters of the human hand. Bioengineering (Basel) 10(3), 324 (2023).
Jull, G. et al. Cervical musculoskeletal impairment in frequent intermittent headache. Part 1: Subjects with single headaches. Cephalalgia 27(7), 793–802 (2007).
Yong, N. et al. Prevalence and risk factors for depression and anxiety among outpatient migraineurs in mainland China. J. Headache Pain 13(4), 303–310 (2012).
Zang, Y. et al. Regional homogeneity approach to fMRI data analysis. Neuroimage 22(1), 394–400 (2004).
Charan, J. & Biswas, T. How to calculate sample size for different study designs in medical research?. Indian J. Psychol. Med. 35(2), 121–126 (2013).
Mingels, S. et al. Exploring multidimensional characteristics in cervicogenic headache: Relations between pain processing, lifestyle, and psychosocial factors. Brain Behav. 11(10), e2339 (2021).
Sedighi, A., Nakhostin, A. N. & Naghdi, S. Comparison of acute effects of superficial and deep dry needling into trigger points of suboccipital and upper trapezius muscles in patients with cervicogenic headache. J. Bodyw. Mov. Ther. 21(4), 810–814 (2017).
Huang, T. et al. Altered spontaneous activity in patients with persistent somatoform pain disorder revealed by regional homogeneity. PLoS One 11(3), e151360 (2016).
Liu, X. et al. CACNA1C gene rs11832738 polymorphism influences depression severity by modulating spontaneous activity in the right middle frontal gyrus in patients with major depressive disorder. Front. Psychiatry 11, 73 (2020).
Medina, S. et al. Regional cerebral blood flow as predictor of response to occipital nerve block in cluster headache. J. Headache Pain 22(1), 91 (2021).
Mohamadi, M. et al. Can the positional release technique affect central sensitization in patients with chronic tension-type headache? A randomized clinical trial. Arch Phys Med Rehabil 101(10), 1696–1703 (2020).
Kim, J. et al. Longitudinal FDG microPET imaging of neuropathic pain: Does cerebellar activity correlate with neuropathic pain development in a rat model?. Acta Neurochir. (Wien) 157(6), 1051–1057 (2015).
Jie, W. & Pei-**, C. Discharge response of cerebellar Purkinje cells to stimulation of C-fiber in cat saphenous nerve. Brain Res. 581(2), 269–272 (1992).
Funding
This work was funded by The Exploration Project of Natural Science Foundation of Zhejiang Province (Grant No. LQ22H270007) and Zhejiang Province Traditional Chinese Medicine Science and Technology Project (Grant No. 2022ZB121).
Author information
Authors and Affiliations
Contributions
H.-G.D. designed the study. S.-J.L. collected the data. K.N. analyzed the data. J.X. wrote the first version. X.J., C.-W.J. revised the manuscript and all other authors added their comments. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
**, X., Du, HG., Kong, N. et al. Clinical efficacy of the mulligan maneuver for cervicogenic headache: a randomized controlled trial. Sci Rep 13, 22034 (2023). https://doi.org/10.1038/s41598-023-48864-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48864-1
- Springer Nature Limited