Abstract
Sacubitril/valsartan (SAC/VAL), an angiotensin receptor blocker-neprilysin inhibitor, has been widely used to treat several types of heart failure. Nevertheless, the effects of drugs in mitral regurgitation patients, from the molecular level to therapeutic effects, remain unclear. This study investigates the roles of SAC/VAL on cardiac function, mitochondrial quality, autophagy, mitophagy, and natriuretic peptides in a rat model of chronic mitral regurgitation. Male Sprague–Dawley rats underwent MR induction (n = 16) and sham surgeries (n = 8). Four weeks post-surgery confirmed MR rats were randomly divided into MR (n = 8) and SAC/VAL (n = 8) groups. The SAC/VAL group was administered SAC/VAL, whereas the MR and the sham rats received vehicle via oral gavage daily for 8 weeks. Cardiac geometry, function, and myocardial fibrosis were assessed by echocardiography and histopathology. Spectrophotometry and real-time PCR were performed to assess the pharmacological effects on mitochondrial quality, autophagy, mitophagy, and natriuretic peptides. MR rats demonstrated significant left heart dilation and left ventricular systolic dysfunction compared with the sham group, which could be significantly improved by SAC/VAL. In addition, SAC/VAL significantly reduced myocardial cardiac remodeling and fibrosis in MR rats. SAC/VAL improved the mitochondrial quality by attenuating mitochondrial reactive oxygen species production and mitochondrial depolarization compared with the MR group. Also, the upregulation of autophagy-related, mitophagy-related, and natriuretic peptide system gene expression in MR rats was attenuated by SAC/VAL treatment. In conclusion, this study demonstrated that SAC/VAL treatment could provide numerous beneficial effects in MR conditions, suggesting that this drug may be an effective treatment for MR.
Similar content being viewed by others
Introduction
Mitral regurgitation is the most prevalent valvular disease in the aging population1, and the most common heart disease found in small-breed, middle-old aged dogs2. The renin–angiotensin–aldosterone system (RAAS) and natriuretic peptide system (NPs) are essential mechanisms that are accelerated as compensatory mechanisms to preserve cardiac output and stabilize haemodynamic status when mitral regurgitation occurs along with its consequences, including left ventricular (LV) volume overload, LV systolic dysfunction, and LV remodeling3,4. However, if MR progression continuously develops with chronic activation of these systems, they will become a vicious cycle, culminating in subsequent heart failure (HF)5. HF is a necessary consequence of MR, resulting in a high and increasing mortality rate worldwide6.
Recently, studies have mentioned several cellular mechanisms contributing to the disease progression through HF. Volume overload and RAAS activation are critical factors that initiate reactive oxygen species (ROS) production, inducing mitochondrial damage and dysfunction and leading to cardiac injury, remodeling, and HF7. During this period, autophagy upregulation is responsible for dysfunctional protein turnover and cell biogenesis preservation8,9. Microtubule-associated protein 1A/1B-light chain 3 (LC3), an indicator of autophagy, is upregulated in MR10 and was significantly associated with the severity of the myolysis in MR11. According to this point of view, novel pharmacological interventions for HF patients are continuously being developed that focus deeper on mechanisms involved with disease pathophysiology12. The drugs that can positively affect these mechanisms will be effective treatments for cardiac patients in the long run.
Sacubitril/valsartan (SAC/VAL) combines an angiotensin II type 1 receptor (AT1R) blocker and a neprilysin inhibitor, ARNi, was approved by the U.S. Food and Drug Administration for heart failure (HF) with reduced ejection fraction treatment in 201513. The dual mechanisms of SAC/VAL are to inhibit the over-activation of pathological RAAS through an AT1R blocker and enhance the salutary effects of NPs by degrading the neprilysin enzyme and having potentially synergistic effects14. Previous studies revealed that SAC/VAL improved cardiac function and limited cardiac remodeling, inflammation, and fibrosis in experimental and clinical studies of cardiovascular diseases14,15,16,49. After the successful creation of MR, the thoracic wall and skin were anatomically closed with suitable suture materials. A similar surgical procedure was performed without the LV and mitral leaflet puncture for the sham operation. The recovering animals were moved to an individual recovery cage where they were provided a warm and dry environment and monitored until they presented completely normal behavior. They were administered diluted enrofloxacin (10 mg/kg, SC) and tramadol (12.5 mg/kg, SID, IP) daily for 5 days after surgery.
The experimental procedures/pharmacological therapy
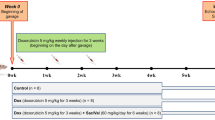
Four weeks after surgery, transthoracic echocardiography was done to assess the severity of the MR-volume overload model focusing on the presence of MR (> 30% of LA area) or LA enlargement (left atrium to the aortic root ratio; LA: Ao > 1.6) in all rats and used as baseline data. The MR rats (n = 16 rats) were randomly assigned to two groups: the MR group (drinking water; n = 8) and the sacubitril/valsartan (SAC/VAL) group (68 mg/kg PO daily; n = 8)14. All rats received the treatments daily via oral gavage for 8 weeks using the appropriate doses in the previous study14. For the sham group, the rats were given the vehicle daily (drinking water) in the same volume as other treatment groups.
Echocardiography
Transthoracic echocardiography was done 4 weeks after surgery (baseline) and at the end of week 8 after the drug administration to evaluate drug effects on cardiac geometry and cardiac function in all groups. The Mindray M9 echocardiographic machine (Mindray, Shenzhen, China) equipped with a P10-4E (4–10 MHz) phase array transducer probe was used to acquire conventional echocardiographic parameters. The rats were mildly sedated with isoflurane, and the fur around the thoracic area was clipped. Then, the rats were placed in the right, lateral recumbent position and electrocardiographic (ECG) electrodes were attached to both forelimbs and the left hindlimb and connected to the machine.
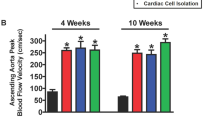
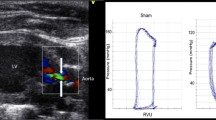
LV conventional echocardiography consists of the interventricular septum and LV posterior wall thickness during systole and diastole (IVSd, IVSs, LVPWd, and LVPWs) and the left ventricular internal dimension end-diastole and end-systole (LVIDd, LVIDs) derived from the M-mode of the right parasternal short-axis view at the head of the papillary muscle level. From this view, the LV FS, LV EF, LV EDV, and LV ESV were calculated using the Teichholz method50,51. A standard two-dimensional (2D) right parasternal short-axis view at the base of the heart was performed to acquire the left atrium to aortic root ratio (LA: Ao). The right parasternal long-axis view with color map** was performed to assess the MR jet area (%), which indicated the severity of MR22. In addition, the aortic flow velocity (AV Vmax) was obtained. All parameters were obtained from an average of six consecutive cardiac cycles.
Tissue collection and fibrosis assessment
At the end of 8 weeks after drug administration, all rats were euthanized with overdose of isoflurane in an induction chamber connected to scavenging system. The heart was instantly harvested and perfused with ice-cold phosphate-buffered saline (PBS). Heart weight was obtained for the HW to BW ratio calculation. The LV-free wall was separated into three segments, similar regions, for further analysis. For the first part, approximately 30 mg of tissue was preserved in RNAlater and kept at − 20 °C for further PCR analysis. In another part, approximately 300 mg of the LV-free wall was immediately used as fresh LV tissue for mitochondrial isolation and mitochondrial quality analyses. The remaining LV section was preserved in 10% buffered formalin and embedded in paraffin. Then, myocardium-embedded blocks were sectioned and stained with hematoxylin and eosin stain (H&E) for histomorphology analyses. In addition, the blocks were sectioned into 5-µm-thick slices and stained with Masson’s trichrome stain to evaluate myocardial collagen and fibrosis. The fibrotic area was quantitatively evaluated from scanning of the whole LV section using NIS-element BR analysis software (Nikon Corporation, Tokyo, Japan): the collagen-stained area/the entire sectioned area × 100 and expressed as a percentage of the fibrotic area52. The left ventricular myocyte cross-sectional area was measured using NIS-element BR analysis software as previously described31. Briefly, each myocyte was identified, and its border was traced and the pixels within the border were calibrated against a scale to measure the area. The CSA data were averaged from 30 different cells in each image.
Evaluation of mitochondrial quality
All processes associated with mitochondrial quality analyses were modified from previous studies53.
Mitochondrial isolation
Fresh LV tissue (approximately 300 mg) was excised and washed in ice-cold PBS solution. Then, the tissue was homogenized in ice-cold isolated buffer (300 mM sucrose, 0.2 mM EGTA, 5 mM TES, pH 7.2: 1 mL per tissue 100 mg). The homogenized tissue solution was centrifuged at 800 × g, 4 °C for 10 min. The supernatant (S1) was transferred to a precooled Eppendorf tube and re-centrifuged at 8000 × g, 4 °C for 10 min. Then, the supernatant (S2) was discarded, and the mitochondrial pellet (P2) was washed by resuspending in an ice-cold isolation buffer again and re-centrifuged at 8000 × g, 4 °C for 10 min. Finally, the isolated cardiac mitochondrial protein was resuspended with respiration buffer (100 mM KCl, 50 mM sucrose, 10 mM HEPES, and 5 mM KH2PO4, pH 7.2), and the mitochondrial protein concentration was determined using a Bradford assay (HiMedia Laboratories, Maharashtra, India) at λ 595 nm. Bovine serum albumin was used as a protein standard. The final concentration of the isolated cardiac mitochondrial stock was diluted to 0.4 mg/mL.
Determination of mitochondrial swelling
Mitochondrial swelling is the condition used to determine the sensitivity to membrane permeability transition (mPT)54. Five μL of respiration buffer and 20 mM CaCl2 were separately added into 2 wells of a 96-well plate, labeling them with and without calcium conditions. The isolated cardiac mitochondrial stock (200 μL) was pipetted into these two wells. The plate was measured to determine the rapid loss of the absorbance at λ 540 nm via the spectrophotometric method at 10- and 30-min time points at room temperature. The maximal mitochondrial (∆OD540 nm) swelling amplitude was calculated from the optical density at 10 min (OD1)–30 min (OD2)55. An increase in the ∆OD540 nm indicated mitochondrial swelling.
Determination of mitochondrial ROS production
Non-fluorescent 2′7′-dichlorofluorescein-diacetate (DCFH-DA), which was hydrolyzed by ROS in mitochondria and changed the form to fluorescent 2′,7′-dichlorohydrofluorescein (DCF), was used to evaluate mitochondrial ROS production56. The isolated cardiac mitochondrial stock was incubated with 2 μM DCFH-DA (prepared from 5 mL of respiratory buffer + 10 μL stock of 1 mM DCFH-DA) at 37 °C in the dark for 30 min. A fluorescence microplate reader with the excitation of λ 485 nm and emission of λ 530 nm was used to determine the fluorescence intensity of DCF, which indicated the increase in ROS production.
Determination of mitochondrial membrane potential changes
The JC-1 dye or 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolecarbocyanine iodide (5 μM) was used in this study for an indirect estimate of the mitochondrial membrane potential change (∆ψm). Five μM of JC-1 (prepared from 5 mL of respiratory buffer + 10 μL stock of 2.5 mM JC-1) was incubated with isolated cardiac mitochondrial stock at 37 °C in the dark for 30 min. When membrane depolarization occurs (∆ψm augmentation), the expression of the red and green fluorescence is altered, leading to a decrease in the red: green ratio54. The red and green fluorescence intensity was measured via a fluorescence microplate reader with the excitation at λ 485 nm and emission at λ 530 nm for red and λ 590 nm for green fluorescein. The results revealed the red: green fluorescence intensity ratio.
Real-time polymerase chain reaction (real-time PCR) analysis
The expression levels of autophagy-related genes (LC3-phosphatidylethanolamine conjugate and p62), mitophagy-related genes (PINK and Parkin), and natriuretic peptides (ANP and BNP) were determined using real-time PCR analysis. The 18S rRNA were selected and used as an internal control in this study. The used primer sequences of these genes were obtained from a previous study in rat cardiomyocytes and are displayed in Table 157,58,59. Total RNA was extracted from the LV tissue using an AccuPure tissue RNA mini kit (AccuBioMed®, New Taipei City, Taiwan) in accordance with the manufacturer’s instructions and reverse transcribed to the first-strand complementary DNA (cDNA) using the iScript™ reverse transcription supermix (BioRad, Hercules, USA). The expression level of the target genes and housekee** genes was detected by real-time PCR analysis using PowerUp™ SYBR™ Green Master Mix in conjunction with QuantStudio™ 5 (Thermo Fisher Scientific, Massachusetts, USA), performing duplicate per reactions. The standard reaction conditions were 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 1 min with a total of 40 cycles. The threshold cycle (CT) of 18S rRNA was used to normalize the CT of the target genes (∆CT). Thereafter, the fold change mRNA expression of the target genes to the control group and the fold change mRNA expression of the target genes to the MR group was calculated using formula 2−∆∆CT60.
Statistical analysis
Statistical analysis was performed using IBM® SPSS® statistics commercial software (SPSS Inc., IL, U.S.A.). Continuous variables are demonstrated as mean ± standard error of mean (SEM). Student t-test was performed to differentiate the difference between the Sham and MR groups at baseline of the study. One-way ANOVA test with Tukey’s correction for multiple comparisons was used to evaluate the pharmacological effects of the drugs among groups. P value < 05 was considered statistically significance.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be available because of privacy or ethical restrictions.
Abbreviations
- ANP:
-
Atrial natriuretic peptide
- AT1R:
-
Angiotensin II type 1 receptor
- BNP:
-
Brain (B-type) natriuretic peptide
- BW:
-
Body wight
- CSA:
-
Cross-sectional area
- EDV:
-
End-diastolic volume
- EF:
-
Ejection fraction
- ESV:
-
End-systolic volume
- FS:
-
Fractional shortening
- HF:
-
Heart failure
- HW:
-
Heart weight
- HW:BW:
-
Heart weight per body weight ratio
- LA:
-
Left atrial
- LA:Ao:
-
Left atrial to aortic root ratio
- LC3:
-
Microtubule-associated protein 1A/1B-light chain 3
- LV:
-
Left ventricular
- LVIDd:
-
Left ventricular internal diameter diastole
- LVIDs:
-
Left ventricular internal diameter systole
- MR:
-
Mitral regurgitation
- NEP:
-
Neprilysin enzyme
- NPs:
-
Natriuretic peptide system
- PINK1:
-
PTEN-induced kinase 1
- RAAS:
-
Renin–angiotensin–aldosterone system
- ROS:
-
Reactive oxygen species
- SAC/VAL:
-
Sacubitril/valsartan
References
Nishimura, R. A. et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 70, 252–289. https://doi.org/10.1016/j.jacc.2017.03.011 (2017).
Menciotti, G. & Borgarelli, M. Review of diagnostic and therapeutic approach to canine myxomatous mitral valve disease. Vet. Sci. https://doi.org/10.3390/vetsci4040047 (2017).
Ayalasomayajula, S., Langenickel, T., Pal, P., Boggarapu, S. & Sunkara, G. Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): A novel angiotensin receptor-neprilysin inhibitor. Clin. Pharmacokinet. 56, 1461–1478. https://doi.org/10.1007/s40262-017-0543-3 (2017).
McMurray, J. J. Clinical practice. Systolic heart failure. N. Engl. J. Med. 362, 228–238. https://doi.org/10.1056/NEJMcp0909392 (2010).
Swedberg, K., Eneroth, P., Kjekshus, J. & Wilhelmsen, L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation 82, 1730–1736. https://doi.org/10.1161/01.cir.82.5.1730 (1990).
Savarese, G. & Lund, L. H. Global public health burden of heart failure. Card. Fail. Rev. 3, 7–11. https://doi.org/10.15420/cfr.2016:25:2 (2017).
Moorjani, N. et al. Effects of left ventricular volume overload on mitochondrial and death-receptor-mediated apoptotic pathways in the transition to heart failure. Am. J. Cardiol. 103, 1261–1268. https://doi.org/10.1016/j.amjcard.2009.01.013 (2009).
Zhou, L., Ma, B. & Han, X. The role of autophagy in angiotensin II-induced pathological cardiac hypertrophy. J. Mol. Endocrinol. 57, R143–R152. https://doi.org/10.1530/JME-16-0086 (2016).
Lee, J., Giordano, S. & Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 441, 523–540. https://doi.org/10.1042/BJ20111451 (2012).
Deroyer, C. et al. New biomarkers for primary mitral regurgitation. Clin. Proteomics 12, 25. https://doi.org/10.1186/s12014-015-9097-2 (2015).
Chen, M. C. et al. Autophagy as a mechanism for myolysis of cardiomyocytes in mitral regurgitation. Eur. J. Clin. Invest. 41, 299–307. https://doi.org/10.1111/j.1365-2362.2010.02410.x (2011).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200. https://doi.org/10.1093/eurheartj/ehw128 (2016).
Ibrahim, N. E. et al. Effect of neprilysin inhibition on various natriuretic peptide assays. J. Am. Coll. Cardiol. 73, 1273–1284. https://doi.org/10.1016/j.jacc.2018.12.063 (2019).
Maslov, M. Y., Foianini, S., Mayer, D., Orlov, M. V. & Lovich, M. A. Synergy between sacubitril and valsartan leads to hemodynamic, antifibrotic, and exercise tolerance benefits in rats with preexisting heart failure. Am. J. Physiol. Heart Circ. Physiol. 316, H289–H297. https://doi.org/10.1152/ajpheart.00579.2018 (2019).
Suematsu, Y. et al. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur. J. Heart Fail. 18, 386–393. https://doi.org/10.1002/ejhf.474 (2016).
Almufleh, A. et al. Ejection fraction improvement and reverse remodeling achieved with Sacubitril/Valsartan in heart failure with reduced ejection fraction patients. Am. J. Cardiovasc. Dis. 7, 108–113 (2017).
**a, Y. et al. LCZ696 improves cardiac function via alleviating Drp1-mediated mitochondrial dysfunction in mice with doxorubicin-induced dilated cardiomyopathy. J. Mol. Cell Cardiol. 108, 138–148. https://doi.org/10.1016/j.yjmcc.2017.06.003 (2017).
McMurray, J. J. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004. https://doi.org/10.1056/NEJMoa1409077 (2014).
Kang, D. H. et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 139, 1354–1365. https://doi.org/10.1161/CIRCULATIONAHA.118.037077 (2019).
Yang, C. C. et al. The therapeutic impact of entresto on protecting against cardiorenal syndrome-associated renal damage in rats on high protein diet. Biomed. Pharmacother. 116, 108954. https://doi.org/10.1016/j.biopha.2019.108954 (2019).
Li, X., Braza, J., Mende, U., Choudhary, G. & Zhang, P. Cardioprotective effects of early intervention with sacubitril/valsartan on pressure overloaded rat hearts. Sci. Rep. 11, 16542. https://doi.org/10.1038/s41598-021-95988-3 (2021).
Kim, K. H. et al. Long-term effects of sildenafil in a rat model of chronic mitral regurgitation: Benefits of ventricular remodeling and exercise capacity. Circulation 125, 1390–1401. https://doi.org/10.1161/CIRCULATIONAHA.111.065300 (2012).
Jungtanasomboon, P. et al. Vericiguat preserved cardiac function and mitochondrial quality in a rat model of mitral regurgitation. Life Sci. 10(328), 121929. https://doi.org/10.1016/j.lfs.2023.121929 (2023).
Pfau, D. et al. Angiotensin receptor neprilysin inhibitor attenuates myocardial remodeling and improves infarct perfusion in experimental heart failure. Sci. Rep. 9, 5791. https://doi.org/10.1038/s41598-019-42113-0 (2019).
Saengklub, N., Pirintr, P., Nampimoon, T., Kijtawornrat, A. & Chaiyabutr, N. Short-term effects of sacubitril/valsartan on echocardiographic parameters in dogs with symptomatic myxomatous mitral valve disease. Front. Vet. Sci. 8, 700230. https://doi.org/10.3389/fvets.2021.700230 (2021).
Torrado, J. et al. Sacubitril/valsartan averts adverse post-infarction ventricular remodeling and preserves systolic function in rabbits. J. Am. Coll. Cardiol. 72, 2342–2356. https://doi.org/10.1016/j.jacc.2018.07.102 (2018).
Cosentino, E. R. et al. Sacubitril/valsartan improves both functional and echocardiographic parameters in patients with chronic heart failure with reduced ejection fraction. Curr. Med. Res. Opin. 35, 9–12. https://doi.org/10.1080/03007995.2019.1576481 (2019).
El Sabbagh, A., Reddy, Y. N. V. & Nishimura, R. A. Mitral valve regurgitation in the contemporary era: Insights into diagnosis, management, and future directions. JACC Cardiovasc. Imaging 11(4), 628–643. https://doi.org/10.1016/j.jcmg.2018.01.009.PMID:29622181 (2018).
Suo, Y. et al. Sacubitril/valsartan improves left atrial and left atrial appendage function in patients with atrial fibrillation and in pressure overload-induced mice. Front. Pharmacol. 10, 1285. https://doi.org/10.3389/fphar.2019.01285 (2019).
Zhao, Y. et al. AHU377+valsartan (LCZ696) modulates renin-angiotensin system (RAS) in the cardiac of female spontaneously hypertensive rats compared with valsartan. J. Cardiovasc. Pharmacol. Ther. 24, 450–459. https://doi.org/10.1177/1074248419838503 (2019).
Corporan, D., Segura, A. & Padala, M. Ultrastructural adaptation of the cardiomyocyte to chronic mitral regurgitation. Front. Cardiovasc. Med. 8, 714–774. https://doi.org/10.3389/fcvm.2021.714774.PMID:34733889;PMCID:PMC8559873 (2021).
Burke, R. M., Lighthouse, J. K., Mickelsen, D. & Small, E. M. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ. Heart Fail. 12(4), e005565. https://doi.org/10.1161/CIRCHEARTFAILURE.118.005565 (2019) (PMID: 30998392; PMCID: PMC6530564).
Ge, Q., Zhao, L., Ren, X. M., Ye, P. & Hu, Z. Y. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Exp. Biol. Med. 244, 1028–1039. https://doi.org/10.1177/1535370219861283 (2019).
von Lueder, T. G. et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: Novel therapeutic concepts to combat heart failure. Circ. Heart Fail. 6, 594–605. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000289 (2013).
Bayes-Genis, A., Nunez, J. & Lupon, J. Sacubitril/valsartan as antifibrotic drug: Rejuvenating the fibrosed HFpEF heart. J. Am. Coll. Cardiol. 76, 515–517. https://doi.org/10.1016/j.jacc.2020.06.016 (2020).
Lee, S. R. et al. Mitochondrial DNA, mitochondrial dysfunction, and cardiac manifestations. Front. Biosci. 21, 1410–1426. https://doi.org/10.2741/4541 (2016).
Zhou, B. & Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Invest. 128, 3716–3726. https://doi.org/10.1172/JCI120849 (2018).
Ahmed, M. I. et al. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60%. J. Am. Coll. Cardiol. 55, 671–679. https://doi.org/10.1016/j.jacc.2009.08.074 (2010).
Moe, G., Konig, A., Liu, P. & Jugdutt, B. I. Selective type 1 angiotensin II receptor blockade attenuates oxidative stress and regulates angiotensin II receptors in the canine failing heart. Mol. Cell Biochem. 317, 97–104. https://doi.org/10.1007/s11010-008-9835-0 (2008).
Narendra, D. P. et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298. https://doi.org/10.1371/journal.pbio.1000298 (2010).
Qiu, Z. et al. **n Fu Kang oral liquid inhibits excessive myocardial mitophagy in a rat model of advanced heart failure. Am. J. Transl. Res. 10, 3198–3210 (2018).
Langenickel, T., Pagel, I., Hohnel, K., Dietz, R. & Willenbrock, R. Differential regulation of cardiac ANP and BNP mRNA in different stages of experimental heart failure. Am. J. Physiol. Heart Circ. Physiol. 278, H1500-1506. https://doi.org/10.1152/ajpheart.2000.278.5.H1500 (2000).
Li, L. Y. et al. Sacubitril/valsartan attenuates atrial electrical and structural remodelling in a rabbit model of atrial fibrillation. Eur. J. Pharmacol. 881, 173120. https://doi.org/10.1016/j.ejphar.2020.173120 (2020).
Nishikimi, T., Maeda, N. & Matsuoka, H. The role of natriuretic peptides in cardioprotection. Cardiovasc. Res. 69, 318–328. https://doi.org/10.1016/j.cardiores.2005.10.001 (2006).
Lin, L. et al. Mas receptor mediates cardioprotection of angiotensin-(1–7) against Angiotensin II-induced cardiomyocyte autophagy and cardiac remodelling through inhibition of oxidative stress. J. Cell Mol. Med. 20, 48–57. https://doi.org/10.1111/jcmm.12687 (2016).
Zablocki, D. & Sadoshima, J. Angiotensin II and oxidative stress in the failing heart. Antioxid. Redox Signal 19, 1095–1109. https://doi.org/10.1089/ars.2012.4588 (2013).
Aisa, Z. et al. Effect of autophagy on myocardial infarction and its mechanism. Eur. Rev. Med. Pharmacol. Sci. 21(16), 3705–3713 (2017) (PMID: 28925470).
National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.) & National Academies Press (U.S.). (National Academies Press, Washington, D.C., 2011).
Corporan, D., Kono, T., Onohara, D. & Padala, M. An image guided transapical mitral valve leaflet puncture model of controlled volume overload from mitral regurgitation in the rat. J. Vis. Exp. https://doi.org/10.3791/61029 (2020).
Reffelmann, T. & Kloner, R. A. Transthoracic echocardiography in rats. Evalution of commonly used indices of left ventricular dimensions, contractile performance, and hypertrophy in a genetic model of hypertrophic heart failure (SHHF-Mcc-facp-Rats) in comparison with Wistar rats during aging. Basic Res. Cardiol. 98, 275–284. https://doi.org/10.1007/s00395-003-0401-3 (2003).
Darbandi Azar, A., Tavakoli, F., Moladoust, H., Zare, A. & Sadeghpour, A. Echocardiographic evaluation of cardiac function in ischemic rats: Value of m-mode echocardiography. Res. Cardiovasc. Med. 3, e22941. https://doi.org/10.5812/cardiovascmed.22941 (2014).
Vaskova, E. et al. Sacubitril/valsartan improves cardiac function and decreases myocardial fibrosis via downregulation of exosomal miR-181a in a rodent chronic myocardial infarction model. J. Am. Heart Assoc. 9, e015640. https://doi.org/10.1161/JAHA.119.015640 (2020).
Thummasorn, S., Kumfu, S., Chattipakorn, S. & Chattipakorn, N. Granulocyte-colony stimulating factor attenuates mitochondrial dysfunction induced by oxidative stress in cardiac mitochondria. Mitochondrion 11, 457–466. https://doi.org/10.1016/j.mito.2011.01.008 (2011).
Kumphune, S., Surinkaew, S., Chattipakorn, S. C. & Chattipakorn, N. Inhibition of p38 MAPK activation protects cardiac mitochondria from ischemia/reperfusion injury. Pharm. Biol. 53, 1831–1841. https://doi.org/10.3109/13880209.2015.1014569 (2015).
Heidari, R. & Niknahad, H. The role and study of mitochondrial impairment and oxidative stress in cholestasis. Methods Mol. Biol. 117–132, 2019. https://doi.org/10.1007/978-1-4939-9420-5_8 (1981).
Li, L. et al. 15-deoxy-Delta 12,14-prostaglandin J2 induces apoptosis of human hepatic myofibroblasts. A pathway involving oxidative stress independently of peroxisome-proliferator-activated receptors. J. Biol. Chem. 276, 38152–38158. https://doi.org/10.1074/jbc.M101980200 (2001).
Huang, J. et al. LC3B, a protein that serves as an autophagic marker, modulates angiotensin II-induced myocardial hypertrophy. J. Cardiovasc. Pharmacol. 66, 576–583. https://doi.org/10.1097/FJC.0000000000000306 (2015).
Luo, J. et al. Allopurinol reduces oxidative stress and activates Nrf2/p62 to attenuate diabetic cardiomyopathy in rats. J. Cell Mol. Med. 24, 1760–1773. https://doi.org/10.1111/jcmm.14870 (2020).
Yu, J. et al. Mitochondrial dynamics modulation as a critical contribution for Shenmai injection in attenuating hypoxia/reoxygenation injury. J. Ethnopharmacol. 237, 9–19. https://doi.org/10.1016/j.jep.2019.03.033 (2019).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. https://doi.org/10.1038/nprot.2008.73 (2008).
Acknowledgements
The authors would like to thank Chulalongkorn University Laboratory Animal Center (CULAC) for an in-kind contribution.
Funding
This study was supported by Chulalongkorn University Graduate School for the 90th anniversary of Chulalongkorn University scholarship to L.T. and A.K. (Funding Number GCUGR1125641038M). This study was also partly supported by the National budget to CULAC (Funding Number 390252002).
Author information
Authors and Affiliations
Contributions
L.T., N.S., and A.K. conceived the project, designed, and performed in vivo animal experiments. L.T., A.K., and S.K.T. analyzed the results. L.T., N.S., P.B., A.K., and S.K. performed mitochondrial quality analysis. L.T. performed RT-PCR. L.T. and Y.P. performed histopathology assessment. L.T. and A.K. drafted the manuscript; N.S., S.K., S.K.T. and A.K. supervised and provided critical review of the manuscript. All authors reviewed and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tantisuwat, L., Saengklub, N., Boonpala, P. et al. Sacubitril/valsartan mitigates cardiac remodeling, systolic dysfunction, and preserves mitochondrial quality in a rat model of mitral regurgitation. Sci Rep 13, 11472 (2023). https://doi.org/10.1038/s41598-023-38694-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38694-6
- Springer Nature Limited