Abstract
Immune checkpoint inhibitors (ICIs) change the prognosis of many cancer patients. With the increasing use of ICIs, immune-related adverse events are occurring, including acute kidney injury (AKI). This study aimed to assess the incidence of AKI during ICI treatment and its risk factors and impact on mortality. Patients treated with ICIs at the First Medical Center of the Chinese PLA General Hospital from January 1, 2014, to December 30, 2019, were consecutively enrolled, and risk factors affecting AKI development in patients treated with ICIs were analyzed using univariate and multivariate logistic regression. Medical record surveys and telephone inquiry were used for follow-up, and Kaplan–Meier survival analysis and Cox regression were used to analyze independent risk factors for death. Among 1615 patients, 114 (7.1%) had AKI. Multivariate logistic regression analysis showed that anemia, Alb < 30 g/L, antibiotic use, diuretic use, NSAID use and proton pump inhibitor use were independent risk factors for AKI development in patients treated with ICIs. Stage 2 or 3 AKI was an independent risk factor for nonrecovery of renal function after AKI onset. Multivariate Cox regression analysis showed that anemia, Alb < 30 g/L, AKI occurrence, and diuretic use were independent risk factors for death in patients treated with ICIs, while high baseline BMI, other tumor types, ACEI/ARB use, and chemotherapy use were protective factors for patient death. AKI occurs in 7.1% of patients treated with ICIs. Anemia, Alb < 30 g/L, and combined medication use are independent risk factors for AKI in patients treated with ICIs. Anemia, Alb < 30 g/L, AKI occurrence, and diuretic use were independent risk factors for death in patients treated with ICIs.
Similar content being viewed by others
Induction
The recent development of immune checkpoint inhibitors (ICIs) has driven a revolutionary change in cancer treatment1. ICIs target immune effector cells, primarily T cells, which can result in an effective immune response against tumor cells2. This new treatment improves the outcomes of human cancer patients, and it is now widely used in an increasing number of tumor types3, such as melanoma, lung cancer, and urothelial and renal cell carcinomas. ICIs act by targeting several immune checkpoints, including cytotoxic T lymphocyte–associated antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1). However, ICIs have side effects, such as colitis, hypophysitis, rash, pneumonitis, hypothyroidism, arthralgia and vitiligo, which are called immune-related adverse events (irAEs)4. This treatment may also bring about nephrotoxicity. Renal toxicity from ICIs is not common in clinical trials5,6,7,8,9,10,11. However, in recent years, irAEs in the kidney have received increasing attention, and a series of renal irAEs have been described in case reports and case series, including acute interstitial nephritis (AIN)12, acute tubular necrosis (ATN)13, and glomerular disease14.
Acute kidney injury (AKI) is a clinical syndrome characterized by the deterioration of renal function that rapidly develops over hours to days15. The acute phase of AKI not only causes disturbances in the internal environment and increases the risk of death but also has important long-term effects on multiple organs, such as the heart, lungs, and brain. AKI is also an important factor in prolonging patients' hospital stays, increasing long-term and short-term mortality, and increasing their hospital costs16. AKI associated with ICIs is rare in clinical trials5,6,7,8,9,10,11, but its incidence in real life may be higher than previously reported. Previously, it was reported in the literature that the incidence of ICI-AKI was 1.4 to 4.9%17,18. In the past two years, with the increasing use of ICIs, recent studies on ICI-AKI have shown that the incidence of ICI-AKI is approximately 14–18%19,20,21,22,23.
Studies have shown that age and baseline renal function are independent risk factors for the occurrence of ICI-induced AKI23. A multicenter study showed that the use of proton pump inhibitors (PPIs) and extrarenal irAEs are both associated with a higher risk of ICI-AKI24. In addition, whether AKI is associated with an increased risk of death is still inconclusive. Research by Alejandro Meraz-Muñoz et al. showed that AKI was not associated with death19, but in the study of Frank B. Cortazar et al., if renal function does not recover in patients with ICI-AKI, it is independently associated with a higher mortality rate25. The study by Clara Garcıá-Carro et al. also found that AKI was a risk factor for death23.
However, there are still relatively few data from studies on AKI during ICI treatment, especially in Asian patients. Given that China has a large cancer population, an increasing number of patients will be treated with ICIs. Our goal is to clarify the incidence and risk factors of AKI during ICI treatment and its effect on mortality in Chinese patients.
Methods
Study design and patient selection
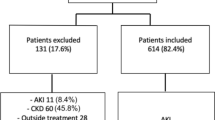
A retrospective study was performed on 1666 patients treated with ICIs who were hospitalized at the Chinese PLA General Hospital from January 2014 to December 2019. The inclusion criteria were as follows: (1) patients were aged ≥ 18 years; (2) patients admitted to the hospital were mainly diagnosed with solid malignant tumors; and (3) patients were hospitalized in our hospital and received at least one dose of the following ICIs: nivolumab, pembrolizumab, atezolizumab, durvalumab, sintilimab, camrelizumab, toripalimab and ipilimumab. The exclusion criteria were as follows: (1) patients with end-stage renal disease (ESRD); (2) patients with a history of nephrectomy or kidney transplantation; and (3) patients with incomplete data during hospitalization and follow-up. Two patients were excluded because they were younger than 18 years, and 49 patients were excluded because they had incomplete medical data (Fig. 1).
Outcome
The primary outcome was the incidence of AKI during treatment with ICIs. AKI was identified and staged by fold-change in creatinine from baseline according to Kidney Disease Improving Global Outcomes (KDIGO) AKI criteria within 12 months of checkpoint inhibitor initiation26,27. Urinary volume was not used as a diagnostic criterion in this study because the measurement of urine volume is influenced by several factors. This study focused on the first episode of AKI after the administration of ICIs. We analyzed the independent risk factors affecting the occurrence of AKI in patients treated with ICIs. We analyzed the independent risk factors for failure to recover renal function in patients with AKI treated with ICIs, and recovery of renal function was defined as recovery of serum creatinine (sCr) to < 1.5 times the baseline value within 90 days after AKI. Because we wanted to examine whether the inability to recover renal function within 90 days after the onset of AKI affected the patient's mortality, we removed the missing values without prognostic follow-up results for the accuracy of the results and did not interpolate.
We also compared mortality in patients with AKI and those without AKI, analyzing risk factors affecting mortality in patients receiving ICIs. The study started follow-up from the time of patient enrollment using a combination of hospital electronic medical records and telephone inquiries, with the endpoint event of patient death or survival and recording the patient's date of death. The enrollment date was the first day of ICI administration. The follow-up cutoff date was September 1, 2021.We lost some data during this 5-year period because many patients treated with ICIs refused to respond to follow-up calls. The follow-up rate was 64.9% (1048/1615). This investigation involved informed consents from all individual participants as well as ethical approval granted by the Ethics Committee in the Chinese PLA General Hospital (Approval No. 2013–050-01).
Clinical collection and definitions
Clinical data were collected from patients treated with ICIs, including age, gender, body mass index (BMI), tumor type, concomitant medications and comorbidities. The estimated glomerular filtration rate (eGFR) was calculated using the eGFR-EPI formula28. Chemotherapeutic medicine included cisplatin, carboplatin, mitomycin, isocyclophosphamide, pemetrexed, cetuximab, methotrexate, and colchicine. Antibiotic medicine included aminoglycoside antibiotics, vancomycin, amphotericin B, rifampin, ciprofloxacin, and sulfonamide antibiotics. The baseline collection time was the baseline value measured closest to the dosing date before the patient first received ICIs, and concomitant medication was the dosing status during the patient's treatment with ICIs. ESRD was defined as eGFR ≤ 15 mL/min/1.73 m2 or the need for renal replacement therapy (RRT). Extrarenal irAEs were recorded based on diagnostic codes and medical records, with concomitant occurrence of AKI. Treatment for AKI and overall outcomes, including survival during follow-up, were documented. All AKI cases were reviewed by two nephrologists, and the causes of AKI were divided into four categories: checkpoint inhibitor-associated AKI, hemodynamic AKI/acute tubular necrosis (ATN), obstructive AKI, and other causes of AKI. Potential checkpoint inhibitor-associated AKI was defined in patients who, after evaluation by two nephrologists, showed no evidence of another possible cause of AKI and were experiencing another irAE20,27. Hemodynamic AKI/ATN included AKI occurring in the setting of dehydration (circulatory collapse, diarrhea, vomiting, etc.), septic or ischemic ATN conditions, and obstructive AKI included all identified causes of bilateral ureteral or urethral orifice obstruction.
Statistical analysis
SPSS 24.0 software was used for statistical analysis. Normally distributed measures are expressed as \(\overline{\rm X}\pm {\rm s}\), and independent samples t test was used for comparison between groups; count data are expressed as number of cases and percentages, and χ2 test was used for comparison between groups. Univariate and multivariate logistic regression analyses were used to identify AKI risk factors; the independent variables included in the logistic regression analysis were selected based on the results of previous studies19,25,27,29,30. We report the odds ratios (ORs) with 95% confidence intervals (CIs) for each covariate of interest. Variables with P values < 0.05 in univariate analysis were selected as independent variables for multivariate analysis. Multivariate logistic regression was used to determine the predictors of renal recovery in patients with ICI-AKI, and the independent variables included in the logistic regression analysis were selected based on the results of previous studies25,30.
Kaplan–Meier curves and multivariate Cox regression models were used to assess the relationship between the occurrence of AKI and the recovery of renal function after the onset of AKI and other factors affecting survival. Hazard ratios (HR) and 95% CIs for mortality were reported. Two-sided P values < 0.05 were considered statistically significant.
Statement
We confirm that all methods were carried out in accordance with relevant guidelines and regulations in methods section.
Results
Characteristics of the study cohort
During the study period, 1615 patients were treated with ICIs at our center and were screened for inclusion in the study. The average age was 57.41 ± 11.59 years; among them, 1115 patients (69.0%) were male, 569 patients (35.2%) had lung cancer, and 354 patients (21.9%) had tumors of the hepatobiliary system (Fig. 2). Anti-PD-1 was the most common type of ICI used (96.7% of patients) (Fig. 2). The baseline sCr level was 70.74 ± 23.61 μmol/L, and the median follow-up time was 26.8 months (Table 1).
Baseline characteristics of patients in the AKI and non-AKI groups
A total of 114 patients (7.1%) with AKI and 1501 patients without AKI were included. The baseline characteristics and distribution of AKI stage are shown in Table 1 (76.3% in stage 1, 16.7% in stage 2 and 7.0% in stage 3). A review of the etiology in all 114 patients with AKI revealed that 6 patients had ICI-AKI (5.2% of all AKI patients). Among patients with non-ICI-related AKI, 35 experienced hemodynamic or prerenal AKI and 11 experienced obstructive AKI. The most frequently used ICI in the AKI patient group was nivolumab, and the most common tumor type was hepatobiliary system malignancy (Fig. 2).
Patients with AKI were similar to those without AKI in terms of age, gender, BMI, drug grou** of ICIs, and chemotherapy drugs taken. Compared to patients in the non-AKI group, patients in the AKI group had lower baseline SCr levels and higher baseline eGFRs. Further analysis revealed that although the AKI group had better baseline renal function, physicians gave a greater proportion of potentially nephrotoxic drugs (PPIs, diuretics, NSAIDs, and antibiotics) to patients with better baseline renal function (Table 1).
Risk factors for AKI in patients receiving ICIs
Univariate logistic analysis showed that baseline eGFR, anemia, baseline albumin (Alb) < 30 g/L, and use of ACEIs/ARBs, antibiotics, diuretics, NSAIDs, PPIs, nivolumab, toripalimab, sintilimab and extrarenal irAEs were associated with the occurrence of AKI (P < 0.05). Statistically significant variables from the univariate analysis were included in the multivariate logistic regression analysis, which showed that anemia (OR: 1.95; 95% CI: 1.16–3.28; P = 0.0123), baseline Alb < 30 g/L (OR: 1.62; 95% CI: 1.17–2.23; P = 0.0034), use of antibiotics (OR: 2.56; 95% CI: 1.10–5.95; P = 0.0288), use of diuretics (OR: 1.75; 95% CI: 1.12–2.73; P = 0.0132), use of NSAIDs (OR: 3.12; 95% CI: 1.96–4.97; P < 0.001), and use of PPIs (OR: 2.07; 95% CI: 1.17–3.66; P = 0.0124) were independent risk factors for the development of AKI (Table 2) (Fig. 4).
Baseline characteristics of patients in the nonsurvivor and survivor groups
At the end of the follow-up, a total of 690 patients died (65.8%), and 358 patients survived (34.2%), with a median follow-up time of 804 days (26.8 months) for all patients. The median time from the first ICI drug to death was 417 days (34.75 months) for patients in the nonsurvivor group. A total of 88 patients had AKI (8.4%); 82 patients in the AKI patient group died, with a mortality rate of 93.2%, and 608 patients in the non-AKI group died, with a mortality rate of 63.3%. Compared with the survivor group, the nonsurvivor group had a lower BMI level, a lower proportion of patients using ACEI/ARB drugs (P < 0.05), and higher proportions of patients with baseline Alb < 30 g/L, anemia and AKI (P < 0.05). There was no significant difference between the two groups in terms of patient age, gender, tumor type, use of chemotherapy drugs, or the proportion of patients classified by the use of ICI drugs (Table 3).
Risk factors for failure to recover renal function in patients with AKI treated with ICIs
Among 88 patients with AKI, 47 patients (53.4%) failed to recover renal function within 90 days after the occurrence of AKI. Factors such as gender, age, BMI, tumor type, baseline SCr, baseline eGFR, AKI stage, and whether hormone therapy was used after the occurrence of AKI were included in the multivariate logistic regression analysis, and the results showed that the occurrence of stage 2 or 3 AKI (OR: 4.06; 95% CI: 1.15–14.37; P = 0.03) was an independent risk factor (Table 4) (Fig. 4).
Risk factors for death in patients treated with ICIs
Univariate Cox regression analysis showed that eGFR, Alb < 30 g/L, anemia, use of antibiotics, use of NSAIDs, occurrence of AKI, and nonrecovery of renal function after the occurrence of AKI were risk factors for death in patients treated with ICIs (P < 0.05) (Fig. 3). Indicators that were statistically significant in the univariate Cox regression analysis were included in the multivariate Cox regression analysis model, and the results of the multivariate Cox regression analysis showed that anemia, Alb < 30 g/L, use of diuretics, and occurrence of AKI were independent risk factors for death in patients treated with ICIs (P < 0.05) (Table 5) (Fig. 4); BMI, use of ACEIs/ARBs, use of chemotherapeutic agents, and other types of tumors were protective factors for death in patients treated with ICIs (P < 0.05) (Table 5) (Fig. 4).
Discussion
ICIs are new anticancer drugs that have revolutionized the natural course of many malignancies, such as melanoma, renal cell carcinoma, non-small-cell lung cancer, bladder cancer, Hodgkin's lymphoma and other malignancies31. Although ICIs have greatly improved the prognosis of patients with malignancies, a number of irAEs have also occurred, one of which is AKI. Initially, the nephrotoxicity of ICIs was often referred to as "creatinine elevation" in some oncology clinical trials32 and was not further investigated. In recent years, however, there has been growing evidence that the occurrence of AKI has a significant impact on the prognosis of patients treated with ICIs.
This is one of the largest published series evaluating AKI in more than 1600 patients treated with ICIs at a single center. First, AKI was found to be a common complication in patients treated with ICIs in this study, which showed an incidence of 7.1% for AKI. In this study, anemia, baseline Alb < 30 g/L, and the use of antibiotics, diuretics, NSAIDs, and PPIs were found to be independent risk factors for develo** AKI in patients treated with ICIs. Second, we followed up patients and found that the risk of death was higher in patients who developed AKI with unrecoverable renal function than in patients with recovered renal function and that the development of stage 2 or 3 AKI was an independent risk factor for unrecoverable renal function after the development of AKI in patients treated with ICIs. Third, further studies found that baseline anemia, baseline Alb < 30 g/L, occurrence of AKI, and use of diuretics were independent risk factors for death in patients; higher baseline BMI, other tumor types, use of ACEIs/ARBs, and use of chemotherapeutic agents were protective factors for patient death.
Univariate and multivariate logistic regression analyses revealed that the use of NSAIDs, antibiotics, and PPIs were independent risk factors for the development of ICI-AKI. This is consistent with the findings of Harish Seethapathy27, Maen Abdelrahim33 and Frank B Cortazar25 et al. Their studies all showed the use of PPIs as a risk factor for ICI-AKI. First, PPIs, NSAIDs and antibiotics can cause acute tubulointerstitial nephritis (ATIN), and the mechanism by which these 3 drugs cause ATIN may be related to their resulting drug-specific T-cell activation in hypersensitivity reactions34,35. Second, treatment with ICIs may lead to loss of tolerance to potentially nephrotoxic drugs by reactivating drug-specific T cells in some patients, lowering the body's tolerance threshold to potentially nephrotoxic drugs36. In our study, we did not find extrarenal irAEs to be associated with the occurrence of AKI by multivariate logistic regression analysis, which differs from the results of previous studies19,30; this difference may be related to the low number of patients with irAEs in our center and possibly to the lack of diagnosis or course of disease documentation for minor irAEs by clinicians; in addition, our study for extrarenal irAEs was concomitant with the occurrence of AKI, which may have contributed to this result.
In this study, 690 patient deaths were recorded at the end of the follow-up, with a very high mortality rate (65.8%), which is similar to the findings of Claire Stein37, Meraz-Muñoz38, and Clara et al23. The mortality rates of patients in their studies ranged from 52.3 to 72.0%. The higher patient mortality results may be attributed to the characteristics of the oncology department in our center, where some patients with refractory malignancies nationwide are referred to our center for treatment because of the high number of RCT studies conducted in our oncology department; on the other hand, ICIs are often used to treat many patients with metastatic and refractory malignancies39, which may also contribute to the higher patient mortality rate at the end of the follow-up of this study. The results of this study showed that the occurrence of AKI was an independent risk factor for death in patients treated with ICIs, which is consistent with the findings of Garcıá-Carro23 and Shruti Gupta24 et al. There are two possible reasons for this result. First, the general condition of patients with AKI is poorer. In our study, the proportions of patients with baseline Alb < 30 g/L and anemia were higher in the AKI group than in the non-AKI group, and both baseline Alb < 30 g/L and anemia were found to be independent risk factors for AKI and death. Lower baseline anemia and lower baseline Alb levels may, to some extent, be markers of poor baseline basal status; second, AKI leads to deterioration of the overall condition of patients, causing disturbance of the internal environment through various pathophysiological mechanisms, which leads to death.
In this study, we studied a subgroup of patients who developed AKI. Forty-seven patients (53.4%) developed AKI and then failed to recover renal function. The development of stage 2 or 3 AKI was an independent risk factor for failure to recover renal function in patients with AKI, which also reflects the importance of the early identification and diagnosis of AKI and aggressive treatment to prevent the progression of stage 1 AKI to stage 2 or 3 AKI. In survival analysis, the risk of death was found to be greater in patients who failed to recover renal function within 90 days after the onset of AKI than in those who recovered renal function, which is consistent with the findings of Cortazar, F. B25 et al. The increased risk of death due to failure to recover renal function after the occurrence of AKI may be because oncologists have very limited options for cancer treatment in some patients with persistent renal impairment, which in turn leads to tumor progression and an increased risk of death; therefore, this reflects the importance of the early recognition and treatment of this adverse event before irreversible renal damage occurs.
It should be specifically noted that the majority of patients included in this study had normal renal function at baseline. This may be because many patients participated in clinical trials at our institution and because many therapeutic trials of cancer drugs exclude patients with CKD and ESRD. Therefore, future studies need to focus more on this group of patients and provide more research data to address the development of ICI-AKI in the CKD population and its impact on kidney prognosis and cancer prognosis. This may become increasingly important as many cancer patients with CKD will become candidates for immunotherapy as the indications for immunologic agents expand.
Although this is the largest study of ICI-AKI conducted in Chinese patients, we acknowledge some limitations. First, not all patients with ICI-AKI in this study underwent renal puncture biopsy and nephrology laboratory tests, such as routine urine tests, so it was not possible to accurately distinguish the specific etiology of AKI. Second, another limitation of this study is that the cause of death of the patients who died was not considered. Third, our outcome analysis focused on renal recovery and overall survival, and no data were collected on tumor response after patients were treated with ICIs. Finally, this study was a single-center retrospective study, and some bias may exist in the analysis.
In conclusion, this study showed that the incidence of ICI-AKI was 7.1%. Anemia, baseline Alb < 30 g/L, and the use of antibiotics, diuretics, NSAIDs, and PPIs were independent risk factors for the development of AKI in patients receiving ICIs. The risk of death was higher in patients with unrecoverable renal function after the occurrence of ICI-AKI than in patients with recovered renal function, and the occurrence of high-grade AKI (stage 2 or 3 AKI) was an independent risk factor for the occurrence of unrecoverable renal function after AKI in patients treated with ICIs. Baseline anemia, baseline Alb < 30 g/L, occurrence of acute kidney injury, and use of diuretics were independent risk factors for death in patients treated with ICIs. Higher baseline BMI, other types of tumors, use of ACEIs/ARBs, and use of chemotherapeutic agents were protective factors against death in patients treated with ICIs.
Data availability
The datasets generated and analysed during the current study are not publicly available due regulations on the Big Data Research Center of the Chinese PLA General Hospital but are available from the corresponding author on reasonable request.
References
Sury, K., Perazella, M. A. & Shirali, A. C. Cardiorenal complications of immune checkpoint inhibitors. Nat. Rev. Nephrol. 14, 571–588. https://doi.org/10.1038/s41581-018-0035-1 (2018).
Hurkmans, D. P. et al. Granzyme B is correlated with clinical outcome after PD-1 blockade in patients with stage IV non-small-cell lung cancer. J. Immunother Cancer. https://doi.org/10.1136/jitc-2020-000586 (2020).
Darvin, P., Toor, S. M., Sasidharan Nair, V. & Elkord, E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 50, 1–11. https://doi.org/10.1038/s12276-018-0191-1 (2018).
Khoja, L., Day, D., Wei-Wu Chen, T., Siu, L. L. & Hansen, A. R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Annals Oncol. Official J. Eur. Soc. Med. Oncol. 28, 2377–2385. https://doi.org/10.1093/annonc/mdx286 (2017).
Barlesi, F. et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): An open-label, randomised, phase 3 study. Lancet Oncol. 19, 1468–1479. https://doi.org/10.1016/s1470-2045(18)30673-9 (2018).
Goldberg, S. B. et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 17, 976–983. https://doi.org/10.1016/s1470-2045(16)30053-5 (2016).
Goldberg, S. B. et al. Pembrolizumab for management of patients with NSCLC and brain metastases: Long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 21, 655–663. https://doi.org/10.1016/s1470-2045(20)30111-x (2020).
Langer, C. J. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 17, 1497–1508. https://doi.org/10.1016/s1470-2045(16)30498-3 (2016).
Malhotra, J. et al. A phase 1–2 study of rovalpituzumab tesirine in combination with nivolumab plus or minus ipilimumab in patients with previously treated extensive-stage SCLC. J. Thorac. Oncol. https://doi.org/10.1016/j.jtho.2021.02.022 (2021).
Massard, C. et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death Ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 34, 3119–3125. https://doi.org/10.1200/JCO.2016.67.9761 (2016).
Middleton, G. et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): A single arm, phase 2 trial. Lancet Respir. Med. 8, 895–904. https://doi.org/10.1016/s2213-2600(20)30033-3 (2020).
Bottlaender, L. et al. Acute interstitial nephritis after sequential ipilumumab—nivolumab therapy of metastatic melanoma. J. Immunother Cancer. 5, 57. https://doi.org/10.1186/s40425-017-0261-2 (2017).
Basnet, S., Dhital, R. & Tharu, B. Acute tubulointerstitial nephritis: A case report on rare adverse effect of Pembrolizumab. Medicina (Kaunas). https://doi.org/10.3390/medicina55050176 (2019).
Mamlouk, O. et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J. Immunother. Cancer. 7, 2. https://doi.org/10.1186/s40425-018-0478-8 (2019).
Demirjian, S. et al. Safety and tolerability study of an intravenously administered small interfering ribonucleic acid (siRNA) post on-pump cardiothoracic surgery in patients at risk of acute kidney injury. Kidney Int. Rep. 2, 836–843. https://doi.org/10.1016/j.ekir.2017.03.016 (2017).
Kane-Gill, S. L. et al. Risk factors for acute kidney injury in older adults with critical illness: A retrospective cohort study. Am J Kidney Dis. 65, 860–869. https://doi.org/10.1053/j.ajkd.2014.10.018 (2015).
Manohar, S. et al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta-analysis. Nephrol Dial Transplant. 34, 108–117. https://doi.org/10.1093/ndt/gfy105 (2019).
Cortazar, F. B. et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 90, 638–647. https://doi.org/10.1016/j.kint.2016.04.008 (2016).
Meraz-Munoz, A. et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: Incidence, risk factors and outcomes. J. Immunother Cancer. https://doi.org/10.1136/jitc-2019-000467 (2020).
Koks, M. S. et al. Immune checkpoint inhibitor-associated acute kidney injury and mortality: An observational study. PLoS One 16, e0252978. https://doi.org/10.1371/journal.pone.0252978 (2021).
Seethapathy, H. et al. Incidence and clinical features of immune-related acute kidney injury in patients receiving programmed cell death Ligand-1 inhibitors. Kidney Int Rep. 5, 1700–1705. https://doi.org/10.1016/j.ekir.2020.07.011 (2020).
Shimamura, Y. et al. Incidence and risk factors of acute kidney injury, and its effect on mortality among Japanese patients receiving immune check point inhibitors: A single-center observational study. Clin. Exp. Nephrol. 25, 479–487. https://doi.org/10.1007/s10157-020-02008-1 (2021).
García-Carro, C. et al. Acute kidney injury as a risk factor for mortality in oncological patients receiving check-point inhibitors. Nephrol Dial Transplant https://doi.org/10.1093/ndt/gfab034 (2021).
Gupta, S. et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother Cancer. https://doi.org/10.1136/jitc-2021-003467 (2021).
Cortazar, F. B. et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: A multicenter study. J. Am. Soc. Nephrol 31, 435–446. https://doi.org/10.1681/ASN.2019070676 (2020).
Kellum, J. A. & Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Critical Care (London, England). 17, 204. https://doi.org/10.1186/cc11454 (2013).
Seethapathy, H. et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin. J. Am. Soc. Nephrol. 14, 1692–1700. https://doi.org/10.2215/CJN.00990119 (2019).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Garcia-Carro, C. et al. Acute kidney injury as a risk factor for mortality in oncological patients receiving checkpoint inhibitors. Nephrol Dial Transplant. 37, 887–894. https://doi.org/10.1093/ndt/gfab034 (2022).
Gupta, S. et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother Cancer. https://doi.org/10.1136/jitc-2021-003467 (2021).
Das, S. & Johnson, D. B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother Cancer. 7, 306. https://doi.org/10.1186/s40425-019-0805-8 (2019).
Franzin, R. et al. The use of immune checkpoint inhibitors in oncology and the occurrence of AKI: Where do we stand?. Front Immunol. 11, 574271. https://doi.org/10.3389/fimmu.2020.574271 (2020).
Abdelrahim, M. et al. Incidence, predictors, and survival impact of acute kidney injury in patients with melanoma treated with immune checkpoint inhibitors: a 10-year single-institution analysis. Oncoimmunology 10, 1927313. https://doi.org/10.1080/2162402x.2021.1927313 (2021).
Nast, C. C. Medication-induced interstitial nephritis in the 21st century. Adv. Chronic Kidney Dis. 24, 72–79. https://doi.org/10.1053/j.ackd.2016.11.016 (2017).
Moledina, D. G. & Perazella, M. A. PPIs and kidney disease: From AIN to CKD. J. Nephrol. 29, 611–616. https://doi.org/10.1007/s40620-016-0309-2 (2016).
Shirali, A. C., Perazella, M. A. & Gettinger, S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am. J. Kidney Dis. 68, 287–291. https://doi.org/10.1053/j.ajkd.2016.02.057 (2016).
Stein, C. et al. Acute kidney injury in patients treated with anti-programmed death receptor-1 for advanced melanoma: A real-life study in a single-centre cohort. Nephrol Dial Transplant. 36, 1664–1674. https://doi.org/10.1093/ndt/gfaa137 (2021).
Meraz-Muñoz, A. et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: Incidence, risk factors and outcomes. J. Immunother Cancer. https://doi.org/10.1136/jitc-2019-000467 (2020).
Suo, A. et al. Anti-PD1-induced immune-related adverse events and survival outcomes in advanced melanoma. Oncologist. 25, 438–446. https://doi.org/10.1634/theoncologist.2019-0674 (2020).
Acknowledgements
We thank the Department of Oncology of the Chinese PLA General Hospital for providing clinical information of the patients.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by National Key research and development (R&D) Program of China (2018YFA0108803) and the Natural Science Foundation of China (NSFC) (82170686).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by M.-S.J., R.W., Z.F., Y.-D.W., Y.W., L.Z., X.-F.S., X.-M.C., K.-L.H., G.-Y.C. The first draft of the manuscript was written by Ming-Su Ji and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ji, MS., Wu, R., Feng, Z. et al. Incidence, risk factors and prognosis of acute kidney injury in patients treated with immune checkpoint inhibitors: a retrospective study. Sci Rep 12, 18752 (2022). https://doi.org/10.1038/s41598-022-21912-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21912-y
- Springer Nature Limited