Abstract
Ewing sarcoma (ES) is a rare disease that lacks a prognostic prediction model. This study aims to develop a nomogram and risk classification system for estimating the probability of overall survival (OS) of patients with ES. The clinicopathological data of ES were collected from the Surveillance, Epidemiology and Final Results (SEER) database from 2010 to 2018. The primary cohort was randomly assigned to the training set and the validation set. Univariate and multiple Cox proportional hazard analyses based on the training set were performed to identify independent prognostic factors. A nomogram was established to generate individualized predictions of 3- and 5-year OS and evaluated by the concordance index (C-index), the receiver operating characteristic curve (ROC), the calibration curve, the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI). Based on the scores calculated with the nomogram, ES patients were divided into three risk groups to predict their survival. A total of 935 patients were identified, and a nomogram consisting of 6 variables was established. The model provided better C-indices of OS (0.788). The validity of the Cox model assumptions was evaluated through the Schönfeld test and deviance residual. The ROC, calibration curve, IDI and NRI indicated that the nomogram exhibited good performance. A risk classification system was built to classify the risk group of ES patients. The nomogram compares favourably and accurately to the traditional SEER tumour staging systems, and risk stratification provides a more convenient and effective tool for clinicians to optimize treatment options.
Similar content being viewed by others
Introduction
Ewing sarcoma (ES) is a primary malignant bone tumour composed of proliferating undifferentiated small round cells1. As the third most common malignant bone tumour after osteosarcoma and chondrosarcoma, the incidence of ES begins to increase in the second decade of life2. Additionally, ES is more common in Caucasian populations and has a slight male predominance (sex ratio of 3:2)3,4. It has been reported that people of African and Asian descent had lower incidence rates (0.2 and 0.8 cases per million, respectively), whereas Pacific Islanders, North African/Middle Eastern and European had higher incidence rates, and the incidence rate of European was as high as 1.5 cases per million4,5. The disease most often presents adjacent to bone, and a quarter arises in soft tissues6,7. The most common site for ES was found on long bones, especially the femur, tibia and humerus, and the recurrence and mortality rates of long bone ES were the second highest among all ES locations, following pelvic ES8. The median overall survival of ES patients is less than 12 months9. Before chemotherapy was introduced, only 10% of ES patients survived10. Due to the progress of multimodal treatments such as surgery, chemotherapy and radiotherapy, the 5-year survival rate of local ES that responds to multimodal therapy has increased to 55–65%11. In contrast, the survival rate of patients with metastases is less than 30% for 5 years12. The lungs (50%), bone (25%), and bone marrow (20%) are the most common sites for metastases, followed by the liver and brain13. Due to the rarity of ES, its prognostic factors are still unclear, and there is no internationally recognized risk stratification scheme for ES patients10. Hence, it is urgent to determine the independent prognostic factors of ES and to accurately stratify ES patients’ risk.
Previous studies reported that ES’s potential clinical prognostic factors included age, race, tumour size, tumour stage and surgery11,14,15. However, these studies included a small number of predictors, which is prone to overfitting or biased fitting, resulting in different conclusions. We aim to combine different prognostic factors and establish a more accurate prognostic model based on larger and more recent samples. As a statistical prognostic model, nomograms are reliable and convenient and are widely used in oncology and medicine15. In this study, we extracted data from the Surveillance, Epidemiology, and End Results (SEER) database from 2010 to 2018 to determine the risk factors for overall survival (OS). A nomogram was established to quantify the survival rate of ES patients, and they were further categorized into three risk groups to predict their survival based on the total prognostic scores calculated by the nomogram16.
Results
Patient characteristics
The data of 1130 ES patients from 2010 to 2018 were extracted from the SEER database, of which 935 patients were included based on the inclusion and exclusion criteria. The primary cohort was randomly assigned to the training set (n = 656, 70%) and the validation set (n = 279, 30%). The clinicopathologic characteristics of patients in the training and validation sets are shown in Table 1 and Table S1.
Survival analysis
Univariate analysis was used to calculate the effect of the included variables on survival outcomes, and the results are shown in Fig. S1. Sex (p = 0.9084), race (p = 0.3779) and brain metastasis (p = 0.9030) were not associated with significant differences in survival. Multiple Cox proportional hazard analysis for the remaining variables demonstrated that young patients (≤ 18 years old), small tumour size (≤ 58 mm), no/unknown bone metastasis, localized tumour stage, and received surgery and chemotherapy were independently linked with better survival, while race, sex, tumour site, the number of primary tumours, marital status, liver metastasis, lung metastasis, brain metastasis and radiotherapy were not associated with significant differences in survival. The results of the multivariate analysis are shown in Table 1 and Table S1.
Nomogram construction and performance
The results of the multivariate analysis shown in Table 1 were utilized to construct the nomogram, which was subsequently used to generate individualized predictions of the 3- and 5-year OS of the ES patients (Fig. 1). The score scale calculation chart at the top was used to evaluate each prognostic factor, the total scores were added, and then, the 3- and 5-year OS were calculated as a guide. The weight of chemotherapy and age were the highest shown in the nomogram, followed by tumour stage and tumour size. None of the covariates were associated with time (Fig. S2). The PH assumption was met by OS (p = 0.20) models, as demonstrated by the Schönfeld test. Figure S3 shows that none of the individually observations were extremely influential.
The C-indices provided by the nomogram (validation and training sets) were higher than those of the SEER tumour staging system (OS: 0.788, 0.767 vs. 0.669, p < 0.01, p < 0.01), indicating that this multivariable model has higher discrimination for predicting the prognosis of ES. The area under the curve (AUC) values of the 3- and 5-year OS for the training and validation sets were 0.803 and 0.787 vs. 0.800 and 0.740, respectively (Fig. 2). The calibration curve, which is shown in Fig. 3 and indicates the predicted probabilities and observed outcomes of the model, demonstrated prominent accordance. The IDI and NRI results showed that the new model was better than SEER tumour stage in terms of predictive performance (Table 2).
Stratifying the risk of patients
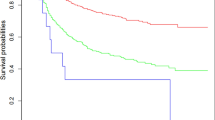
Based on the nomogram, the total prognostic score of each patient was calculated, and patients in the training set were divided into three risk groups to estimate the probability of their OS in accordance with the cut-off points detected by X-tile (Fig. 4A–C). In the validation set, the Kaplan–Meier (KM) curves of OS demonstrated that the risk stratification was stable for predicting the probability of patient survival (Fig. 4D).
Discussion
Ewing sarcoma is a highly malignant bone tumour with poor prognosis. However, due to its low incidence (less than 3 per 1,000,000), its prognostic factors are still controversial17. Current limitations among the different studies focus on the following three aspects: inadequate and old cases, the included variables were insufficient, and the model performed poorly and not validated fully. Hence, the conclusions vary widely between studies. A nomogram is a widely accepted prognostic model that integrates various prognostic factors to predict individual survival. This study extracted the latest ES data from 2010 to 2018 from the SEER database. The included variables in our study were based on previous studies to make the conclusions more representative. The prognostic factors were determined through univariate and multiple Cox proportional hazard analyses. Six independent prognostic factors were identified, and a nomogram was constructed to effectively and intuitively estimate 3- and 5-year OS. To the best of our knowledge, our nomogram provides an improved C-index compared with current studies and shows good discrimination and calibration.
Young age is generally considered to be associated with a better prognosis, while it has a higher incidence in people under the age of 30, especially in children and adolescents18. This might be because adult patients received few cases of chemotherapy, and older patients were more likely to have multiple comorbidities, including diabetes, high blood pressure, and secondary cancer19. Additionally, ES patients above 18 years old are more prone to metastasize at initial diagnosis20. A previous study reported that larger tumours were associated with the risk of recurrence and metastasis21. The metastasis risk can be up to triple with a tumour size greater than 118 mm22. Our research defined 58 mm and 101 mm as two cut-off points and found that tumour sizes between 59 and 101 mm were similar to unknown sizes. The results also showed that bone metastasis and tumour stage were important prognostic factors. Indeed, ES is an invasive type of tumour, with 25% of ES arising in soft tissues rather than bone, and approximately 20–32% of ES patients have distant metastasis11,23. The lungs are the most common site, followed by bone24. Interestingly, lung metastasis was not included in our nomogram, which might be because there was a potential correlation between distant tumour stage and lung metastasis. This further proved the importance of timely diagnosis. However, ES accounts for less than 1% of all cancer diagnoses each year, patients show delayed, nonspecific symptoms similar to common musculoskeletal injuries, and doctors usually have low suspicions25. It is worth noting that our research showed that surgery and chemotherapy were independently associated with OS, while radiotherapy was not found to be an independent prognostic factor. Previous studies reported that when there was no long-term risk of disability, surgery was usually recommended, chemotherapy was also a standard approach for initial treatment, and radiotherapy was only advised for inoperable lesions19,26,27. Through the application of multimodality approaches, the long-term survival rate of localized ES has improved by more than 50% over the last 30 years, while only 20% of ES patients with metastasis can survive for a long time28. Although the survival of ES patients has improved, only 55% of patients received appropriate therapy, which means many therapies were ineffective or unnecessary and hence led to serious late effects. For example, ES is considered radiation-sensitive, while radiotherapy has been controversial, and the proportion of patients who receive radiation alone has been steadily declining. This may be attributed to advances in orthopaedic surgery and chemotherapy and the late effects of radiation in children, such as second malignancies and growth disturbances10.
Our study also concluded that race, sex, tumour site, tumour number, marital status, brain and liver metastasis, and radiotherapy were not found to be independently associated with OS. In particular, race, sex and brain metastasis were excluded after the univariate analysis. Although ES is much more common in white populations and has a slight male predominance29, a previous study reported that ES patients’ morbidity and mortality were not closely related to race or sex2. Although it has been reported that axial tumours are more likely to metastasize at the time of diagnosis, the tumour site was not included in our study, which might be because it has a potential correlation with tumour stage30,31. The primary tumour number was seldom reported before, and we identified that it was not associated with ES, while the reason behind it remained unclear. Marital status was considered a nonindependent factor and may be related to age in ES, as ES is more common in children and adolescents15,16. Brain or liver metastasis was also not identified with a higher risk of death, possibly because few cases had metastasis to these two sites (less than 1.1%).
Finally, we divided the ES patients into three risk groups to predict their survival. As a small round cell malignant tumour, ES presents a similar morphology, which means it is difficult to distinguish the histology grade32,33. Additionally, owing to uniformly poor prognosis, there is no internationally recognized risk classification reference for patients with ES thus far10. Based on the nomogram, we developed a risk stratification scheme to predict the ES' OS and validated it with the validation set.
There were several limitations to this study. First, there was inevitably bias in retrospective studies, and large randomized controlled trials are needed. Second, there were several insufficient prognostic factors in the SEER database, such as genotype and tumour markers. Finally, data collected from other sources were deficient for external verification.
In conclusion, age at diagnosis, tumour size, bone metastasis, tumour stage, surgery and chemotherapy were identified as independent prognostic factors for ES. Based on these independent prognostic factors, a nomogram for OS was constructed. The nomogram provided an improved C-index compared with current studies and showed good discrimination and calibration. Based on the nomogram, ES patients were divided into three risk groups to predict their survival. More research is needed to determine whether it applies to other patient groups.
Methods
Data source and selection
Data from patients diagnosed with ES were extracted from the SEER database, which includes 18 population-based cancer registries covering 30% of the US population34. The SEER database does not provide case identification information, and patient consent is not required to use these data. The research methods were carried out in accordance with relevant guidelines and regulations. The data of ES patients were extracted according to the following criteria: (i) diagnosed with ES based on ICD-O-3 (Third Edition of the International Classification of Diseases for Oncology); (ii) histological confirmation; and (iii) patients with unknown tumour stage, tumour metastasis and race were excluded.
Variables
The demographic variables of the patients who needed to be collected included age at diagnosis, race, sex, tumour site, number of primary tumours, marital status, tumour size, bone metastasis, brain metastasis, lung metastasis, liver metastasis, tumour stage (based on SEER Extent of Disease following a SEER algorithm), surgery, radiotherapy, chemotherapy, vital status and survival months. Age at diagnosis was stratified into three groups, while tumour size was stratified into four groups using the X-tile (Yale University, New Haven, CT, USA) program to obtain the best cut-off points (Fig. 5)35. The primary tumour site was divided into appendix (bones of limb and associated joints) and axial (mandible, vertebral column, rib, sternum, clavicle, pelvic bones, sacrum, coccyx and associated joints) regions. The tumour stage was divided into localized, regional, and distant. The tumour, which was confined entirely to the organ of origin, was defined as localized. Tumours that extended into surrounding organs or tissues were defined as regional. Tumours that spread to parts of the body remote from the primary tumour were defined as distant.
Statistical analysis
Univariate and multiple Cox proportional hazard analyses were used to determine all independent risk factors, and a prognostic nomogram of OS for 3 and 5 years was constructed. The maximum score of each factor in the nomogram was 100 scores. By using the X-tile program, patients were divided into three risk groups based on the nomogram prognostic score, and their survival rates were predicted. The proportional hazards (PH) assumption was checked using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. The deviance residuals were adopted to test influential observations. The concordance index (C-index), the receiver operating characteristic curve (ROC) and the calibration curve were used to analyse the capability of the nomogram. The calibration curve received 1,000 bootstrap repeats and then compared them with the actual survival time. In addition, by calculating the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI), the model’s discrimination was compared with the SEER tumour stage.
Official SEER*Stat software (Version 8.3.9; NCI, Bethesda, USA) was used to collect data36. All statistical analyses were performed by R software version 4.11 (http://www.r-project.org/). The R packages used in this study included rms, survival, foreign, caret, survivalROC, survC1 and survIDINRI. A two-sided p value < 0.05 was considered statistically significant.
References
Mizoshiri, N. et al. Limb saving surgery for Ewing’s sarcoma of the distal tibia: A case report. BMC Cancer 18, 503. https://doi.org/10.1186/s12885-018-4372-z (2018).
Shi, J., Yang, J., Ma, X. & Wang, X. Risk factors for metastasis and poor prognosis of Ewing sarcoma: A population based study. J. Orthop. Surg. Res. 15, 88. https://doi.org/10.1186/s13018-020-01607-8 (2020).
Grunewald, T. G. P. et al. Ewing sarcoma. Nat. Rev. Dis. Primers 4, 5. https://doi.org/10.1038/s41572-018-0003-x (2018).
Ewing sarcoma. Nat. Rev. Dis. Primers 4, 6. https://doi.org/10.1038/s41572-018-0007-6 (2018).
Spector, L. G. et al. Comparative international incidence of Ewing sarcoma 1988 to 2012. Int. J. Cancer 149, 1054–1066. https://doi.org/10.1002/ijc.33674 (2021).
Campbell, K., Shulman, D., Janeway, K. A. & DuBois, S. G. Comparison of epidemiology, clinical features, and outcomes of patients with reported Ewing sarcoma and PNET over 40 years justifies current WHO classification and treatment approaches. Sarcoma 2018, 1712964. https://doi.org/10.1155/2018/1712964 (2018).
Grier, H. E. The Ewing family of tumors. Ewing’s sarcoma and primitive neuroectodermal tumors. Pediatr. Clin. N. Am. 44, 991–1004. https://doi.org/10.1016/s0031-3955(05)70541-1 (1997).
Mounessi, F. S. et al. Pelvic ewing sarcomas. Three-dimensional conformal vs intensity-modulated radiotherapy. Strahlenthe. Onkol. 189, 308–314. https://doi.org/10.1007/s00066-012-0304-z (2013).
Italiano, A. et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 21, 446–455. https://doi.org/10.1016/S1470-2045(19)30825-3 (2020).
Balamuth, N. J. & Womer, R. B. Ewing’s sarcoma. Lancet Oncol. 11, 184–192. https://doi.org/10.1016/S1470-2045(09)70286-4 (2010).
Zhou, Q., Wu, Z. Y. & Lin, Z. Q. A nomogram to predict prognosis in Ewing sarcoma of bone. J. Bone Oncol. 15, 100223. https://doi.org/10.1016/j.jbo.2019.100223 (2019).
Gaspar, N. et al. Ewing sarcoma: Current management and future approaches through collaboration. J. Clin. Oncol. 33, 3036–3046. https://doi.org/10.1200/JCO.2014.59.5256 (2015).
Ladenstein, R. et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J. Clin. Oncol. 28, 3284–3291. https://doi.org/10.1200/JCO.2009.22.9864 (2010).
Zhou, Z., Wang, J., Fang, L., Ma, J. & Guo, M. A nomogram for predicting overall survival in patients with Ewing sarcoma: A SEER-based study. BMC Musculoskelet. Disord. 21, 737. https://doi.org/10.1186/s12891-020-03706-3 (2020).
Zhang, J. et al. A nomogram for determining the disease-specific survival in Ewing sarcoma: A population study. BMC Cancer 19, 667. https://doi.org/10.1186/s12885-019-5893-9 (2019).
Zhang, J. et al. Development and validation of a nomogram containing the prognostic determinants of chondrosarcoma based on the surveillance, epidemiology, and end results database. Int. J. Clin. Oncol. 24, 1459–1467. https://doi.org/10.1007/s10147-019-01489-9 (2019).
Esiashvili, N., Goodman, M. & Marcus, R. B. Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance epidemiology and end results data. J. Pediatr. Hematol. Oncol. 30, 425–430. https://doi.org/10.1097/MPH.0b013e31816e22f3 (2008).
Verma, V., Denniston, K. A., Lin, C. J. & Lin, C. A comparison of pediatric vs. adult patients with the ewing sarcoma family of tumors. Front. Oncol. 7, 82. https://doi.org/10.3389/fonc.2017.00082 (2017).
Wolden, S. L. & Alektiar, K. M. Sarcomas across the age spectrum. Semin. Radiat. Oncol. 20, 45–51. https://doi.org/10.1016/j.semradonc.2009.09.003 (2010).
Ye, C., Dai, M. & Zhang, B. Risk factors for metastasis at initial diagnosis with Ewing sarcoma. Front. Oncol. 9, 1043. https://doi.org/10.3389/fonc.2019.01043 (2019).
Leavey, P. J. et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 51, 334–338. https://doi.org/10.1002/pbc.21618 (2008).
Ramkumar, D. B., Ramkumar, N., Miller, B. J. & Henderson, E. R. Risk factors for detectable metastatic disease at presentation in Ewing sarcoma—An analysis of the SEER registry. Cancer Epidemiol. 57, 134–139. https://doi.org/10.1016/j.canep.2018.10.013 (2018).
Siegel, R. D., Ryan, L. M. & Antman, K. H. Adults with Ewing’s sarcoma. An analysis of 16 patients at the Dana-Farber Cancer Institute. Am. J. Clin. Oncol. 11, 614–617 (1988).
Arpaci, E. et al. Prognostic factors and clinical outcome of patients with Ewing’s sarcoma family of tumors in adults: Multicentric study of the Anatolian Society of Medical Oncology. Med. Oncol. 30, 469. https://doi.org/10.1007/s12032-013-0469-z (2013).
Ferguson, J. L. & Turner, S. P. Bone cancer: Diagnosis and treatment principles. Am. Fam. Phys. 98, 205–213 (2018).
Mukherjee, D. et al. Association of extent of local tumor invasion and survival in patients with malignant primary osseous spinal neoplasms from the surveillance, epidemiology, and end results (SEER) database. World Neurosurg. 76, 580–585. https://doi.org/10.1016/j.wneu.2011.05.016 (2011).
Donaldson, S. S. et al. A multidisciplinary study investigating radiotherapy in Ewing’s sarcoma: End results of POG #8346. Int. J. Radiat. Oncol. Biol. Phys. 42, 125–135. https://doi.org/10.1016/s0360-3016(98)00191-6 (1998).
Rodriguez-Galindo, C. et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann. Oncol. 19, 814–820. https://doi.org/10.1093/annonc/mdm521 (2008).
Parkin, D. M., Stiller, C. A. & Nectoux, J. International variations in the incidence of childhood bone tumours. Int. J. Cancer 53, 371–376. https://doi.org/10.1002/ijc.2910530305 (1993).
Rodriguez-Galindo, C. et al. Analysis of prognostic factors in Ewing sarcoma family of tumors: Review of St. Jude Children’s Research Hospital studies. Cancer 110, 375–384. https://doi.org/10.1002/cncr.22821 (2007).
Karski, E. E. et al. Identification of discrete prognostic groups in ewing sarcoma. Pediatr. Blood Cancer 63, 47–53. https://doi.org/10.1002/pbc.25709 (2016).
de Alava, E. Ewing sarcoma, an update on molecular pathology with therapeutic implications. Surg. Pathol. Clin. 10, 575–585. https://doi.org/10.1016/j.path.2017.04.001 (2017).
Renzi, S., Anderson, N. D., Light, N. & Gupta, A. Ewing-like sarcoma: An emerging family of round cell sarcomas. J. Cell. Physiol. 234, 7999–8007. https://doi.org/10.1002/jcp.27558 (2019).
Lin, Z., Yan, S., Zhang, J. & Pan, Q. A nomogram for distinction and potential prediction of liver metastasis in breast cancer patients. J. Cancer 9, 2098–2106. https://doi.org/10.7150/jca.24445 (2018).
Camp, R. L., Dolled-Filhart, M. & Rimm, D. L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 10, 7252–7259. https://doi.org/10.1158/1078-0432.CCR-04-0713 (2004).
Surveillance Research Program. Database name: Incidence - SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000-2018). National Cancer Institute SEER*Stat Software, Version 8.3.9. www.seer.cancer.gov/seerstat. Accessed August 2021.
Acknowledgements
This study relied entirely on publicly available data in the SEER database and open source initiatives such as the R Project. The authors are grateful to all these projects and all patients who elected to share their data with the community.
Author information
Authors and Affiliations
Contributions
Y.Z., J.L., Y.Q. conceived the concept and designed the study; Y.Z., Z.S., Z.W. performed the experiments; Y.Z., J.L. analysed the data and prepared the figures; Y.Z., Y.Q. wrote the paper; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, Y., Lu, J., Shuai, Z. et al. A novel nomogram and risk classification system predicting the Ewing sarcoma: a population-based study. Sci Rep 12, 8154 (2022). https://doi.org/10.1038/s41598-022-11827-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11827-z
- Springer Nature Limited