Abstract
The heterogeneity of MRI is one of the major reasons for decreased performance of a radiomics model on external validation, limiting the model’s generalizability and clinical application. We aimed to establish a generalizable radiomics model to predict meningioma grade on external validation through leveraging Cycle-Consistent Adversarial Networks (CycleGAN). In this retrospective study, 257 patients with meningioma were included in the institutional training set. Radiomic features (n = 214) were extracted from T2-weighted (T2) and contrast-enhanced T1 (T1C) images. After radiomics feature selection, extreme gradient boosting classifiers were developed. The models were validated in the external validation set consisting of 61 patients with meningiomas. To reduce the gap in generalization associated with the inter-institutional heterogeneity of MRI, the smaller image set style of the external validation was translated into the larger image set style of the institutional training set using CycleGAN. On external validation before CycleGAN application, the performance of the combined T2 and T1C models showed an area under the curve (AUC), accuracy, and F1 score of 0.77 (95% confidence interval 0.63–0.91), 70.7%, and 0.54, respectively. After applying CycleGAN, the performance of the combined T2 and T1C models increased, with an AUC, accuracy, and F1 score of 0.83 (95% confidence interval 0.70–0.97), 73.2%, and 0.59, respectively. Quantitative metrics (by Fréchet Inception Distance) showed that CycleGAN can decrease inter-institutional image heterogeneity while preserving predictive information. In conclusion, leveraging CycleGAN may be helpful to increase the generalizability of a radiomics model in differentiating meningioma grade on external validation.
Similar content being viewed by others
Introduction
Meningiomas are the most common primary intracranial neoplasms in adults, accounting for approximately one-third of all intracranial tumors1. The majority of meningiomas (80%) are classified as low-grade (World Health Organization [WHO] grade 1; benign) and have an indolent clinical course2. On the other hand, high-grade (WHO grade 2 or 3; atypical or anaplastic) tumors have an aggressive biological behavior, a tendency to recur, and a poor prognosis2. The standard management typically involves surgical resection, and adjuvant radiation therapy is often recommended for high-grade meningiomas3. Therefore, develo** a noninvasive generalizable model based on MRI to predict meningioma grade may assist clinical decision making by providing information on treatment planning, including surgical resection strategy4, and care of incidentally detected meningiomas in asymptomatic patients3.
MRI is the key imaging modality for diagnosis and characterization of meningioma and treatment decision5. Several studies applying radiomics, which translates radiological images into high-dimensional mineable imaging data6, have shown promising results in predicting meningioma grade7,8,9,10,11,12. However, majority of them did not perform external validation7. Those studies that performed external validation showed drastically decreased performance in external validation10,11,12, which limits the real-world application of radiomics models. Given that the objective of a prediction model is to predict outcomes in future patients, not to classify previously described characteristics, model generalizability on external validation is critical for model implementation13.
The inter-institutional heterogeneity of MRI protocol is a major reason for decreased performance of a radiomics model in the external validation stage13. Although consensus recommendations for standardized imaging protocol are established in brain tumors such as glioma or brain metastases14,15, consensus imaging protocol for meningiomas is currently lacking, which leads to substantial inter-institutional heterogeneity.
Recently, an approach based on the unpaired image-to-image translation using Cycle-Consistent Adversarial Networks (CycleGAN), a style transfer technique, has been suggested as a promising strategy to overcome poor model performance when dealing with external images16. CycleGAN can transfer the style of the image, while preserving the semantic information within the data16. The approaches using CycleGAN show superior visual similarities between image domains both quantitatively and qualitatively compared with other normalization methods and eliminate manual preparation of the representative reference image because they learn the whole image distribution17,18. We hypothesized that this approach can be applied to convert heterogeneous MRIs and lead to improved performance of a radiomics model to predict meningioma grade on external validation17,18. Thus, the objective of this study was to establish a generalizable radiomics model to predict meningioma grade on external validation through leveraging CycleGAN.
Materials and methods
Patient population
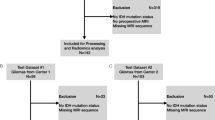
The Yonsei University Institutional Review Board approved this retrospective study and waived the need for obtaining informed patient consent. All methods were performed in accordance with the relevant guidelines and regulations. We identified 297 patients who were pathologically confirmed as having meningioma and underwent baseline conventional MRI between February 2008 and September 2018 in the institutional dataset. Patients with 1) missing MRI sequences or inadequate image quality (n = 17), 2) a previous history of surgery (n = 15), 3) a history of tumor embolization or gamma knife surgery before MRI exam (n = 5), and 4) an error in image processing (n = 2) were excluded. A total of 257 patients (low-grade, 162; high-grade, 95) were enrolled in the institutional cohort.
Identical inclusion and exclusion criteria were applied to identify 62 patients (low-grade, 47; high-grade, 15) from Ewha Mokdong University Hospital between January 2016 and December 2018 for external validation of the model. Patient flowchart is shown in Fig. S1.
Pathological diagnosis
Pathological diagnosis was performed by neuropathologists, according to the WHO criteria19. The criteria for atypical meningioma (WHO grade 2) comprised 4–19 mitoses per 10 high-power fields, the presence of brain invasion, or the presence of at least three of the following features: “sheet-like” growth, hypercellularity, spontaneous necrosis, large and prominent nucleoli, and small cells. The criteria for anaplastic meningioma (WHO grade 3 comprised frank anaplasia (histology resembling carcinoma, sarcoma, or melanoma) or elevated mitoses (> 20 mitoses per 10 high-power fields)19.
MRI protocol
In the institutional training dataset, patients were scanned on 3.0 Tesla MRI units (Achieva or Ingenia; Philips Medical Systems). Imaging protocols included T2-weighted (T2) and contrast-enhanced T1-weighted imaging (T1C). T1C images were acquired after administration of 0.1 mL/kg of gadolinium-based contrast material (Gadovist; Bayer).
In the external validation sets, patients were scanned on 1.5 or 3.0 Tesla MRI units (Avanto; Siemens, or Achieva; Philips Medical Systems), including T2 and T1C images. T1C images were acquired after administration of 0.1 mL/kg of gadolinium-based contrast material (Dotarem; Guerbert, or Gadovist; Bayer). Substantial variation existed between the acquisition parameters for T2 and T1C among the various MRI units between the institutional and external validation sets and reflected the heterogeneity of meningioma imaging data in clinical practice (Supplementary Table 1).
Image preprocessing and radiomics feature extraction
Image resampling to 1-mm isovoxels, low-frequency intensity non-uniformity correction by the N4 bias algorithm, and co-registration of T2 images to T1C images were performed using Advanced Normalization Tools (ANTs)20. After skull strip** by Multi-cONtrast brain STRip** (MONSTR)21, signal intensities were z-score normalized. An affine registration was performed to transform the brain images to the MNI15222.
A neuroradiologist (with 9 years of experience) who was blinded to the clinical information semi-automatically segmented the entire tumor (including cystic or necrotic changes) on the T1C images using 3D Slicer software (v. 4.13.0; www.slicer.org) with edge- and threshold-based algorithms. Another neuroradiologist (with 16 years of experience) re-evaluated and confirmed the segmented lesions.
Radiomic features were calculated with a python-based module (PyRadiomics, version 2.0)23, with a bin size of 32. They included (1) 14 shape features, (2) 18 first-order features, and 3) 75 s-order features (including gray-level co-occurrence matrix, gray-level run-length matrix, gray-level size zone matrix, gray-level dependence matrix, and neighboring gray tone difference matrix) (Supplementary Material S1 and Supplementary Table 2). The features adhered to the standard sets by the Image Biomarker Standardization Initiative 37. However, classical preprocessing steps, such as isovoxel resampling, bias field correction, and signal intensity normalization, are insufficient to counter image heterogeneity. We speculate that CycleGAN may be a practical approach to solve the image heterogeneity of an external dataset. A recent study has shown that CycleGAN can reduce the heterogeneity between radiomic features and increase reproducibility in chest radiographs, which is in line with our study38.
A notable finding in our study was that the T2 radiomics model showed relatively less decreased performance in the external validation set before applying CycleGAN, whereas the T1 radiomics model showed a larger decrease in performance in the external validation set before applying CycleGAN. Compared with the T1C protocols with different protocols, T2 protocols are relatively similar between institutions and less prone to failures from image acquisition artifacts39, which may lead to higher performance on external validation than the T1C model. Nonetheless, after CycleGAN application, the combined T2 and T1C models showed the highest performance. This finding suggests that CycleGAN may preserve the biological information from T2 and T1C sequences while effectively removing inter-institutional variation. Our results are in concordance with other studies that demonstrate that single sequence models have limited ability to reflect the underlying pathophysiology of meningiomas9,40.
Our external validation dataset included different scanner vendors, acquisition protocols, image reconstruction algorithms, and field strengths, resulting in large heterogeneity, which reflects the real-world clinical dataset in meningiomas41. Apart from the different MRI vendors with different field strengths, the resolution, sequence, echo time, repetition time, and inversion time have also not reached consensus in meningioma imaging. All of these differences induce heterogeneity of the MRI datasets, which poses as a unique challenge in the generalizability of the artificial intelligence in this area. Collecting heterogeneous labeled data from multiple institutions worldwide is the best solution to overcome this challenge. Nonetheless, even if we tackle this daunting challenge, the generalizability of the resulting artificial intelligence model cannot be fully guaranteed, as the data in another institution are possibly out-of-distribution. In this study, we demonstrate that leveraging an image harmonizing technique based on deep learning is feasible to increase generalizability in radiomics application for grading meningiomas.
The FID score was lowest in the “original external validation vs. transferred external validation,” rather than in the “training vs. transferred external validation” datasets. Considering the equation in FID42, which calculates the difference between the synthetic and real data distributions, the transferred external validation dataset has understandably the most close resemblance to the original external validation dataset. Nonetheless, the FID score from the “original external validation vs. transferred external validation” datasets decreased to 52.2% compared with that from the “training vs. original external validation” datasets. This result demonstrates that the data distributions between the training and external validation sets became more similar after applying CycleGAN.
This study has several limitations. First, it was conducted with a relatively small amount of data, particularly in the external validation set. As this is a technical feasibility study, a larger multi-institutional validation set is warranted to demonstrate significant performance improvement with CycleGAN. Second, we used two-dimensional CycleGAN rather than three-dimensional CycleGAN because of relative paucity of data. This may lead to slice-to-slice inconsistencies, which may adversely affect the performance. However, despite these limitations, as shortfall in generalization to real-world datasets with heterogeneous imaging data is the major barrier for the adoption of artificial intelligence in medical imaging, the strength of our study is that we demonstrated that CycleGAN is a feasible approach to tackle this challenging issue.
In conclusion, CycleGAN is potentially helpful in increasing the generalizability of a radiomics model in differentiating meningioma grade on external validation.
Abbreviations
- AUC:
-
Area under the curve
- CycleGAN:
-
Cycle-Consistent Adversarial Networks
- FID:
-
Fréchet Inception Distance
- GAN:
-
Generative adversarial network
- T1C:
-
Postcontrast T1-weighted image
- T2:
-
T2-weighted image
- t-SNE:
-
T-Distributed Stochastic Neighbor Embedding
- WHO:
-
World Health Organization
- XGBoost:
-
Extreme gradient boosting
References
Ostrom, Q. T. et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 18, v1–v75. https://doi.org/10.1093/neuonc/now207 (2016).
Kshettry, V. R. et al. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro Oncol. 17, 1166–1173. https://doi.org/10.1093/neuonc/nov069 (2015).
Goldbrunner, R. et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17, e383-391. https://doi.org/10.1016/s1470-2045(16)30321-7 (2016).
Modha, A. & Gutin, P. H. Diagnosis and treatment of atypical and anaplastic meningiomas: A review. Neurosurgery 57, 538–550. https://doi.org/10.1227/01.neu.0000170980.47582.a5 (2005).
Nowosielski, M. et al. Diagnostic challenges in meningioma. Neuro Oncol. 19, 1588–1598. https://doi.org/10.1093/neuonc/nox101 (2017).
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 278, 563–577. https://doi.org/10.1148/radiol.2015151169 (2016).
Won, S. Y. et al. Quality assessment of meningioma radiomics studies: Bridging the gap between exploratory research and clinical applications. Eur J Radiol 138, 109673. https://doi.org/10.1016/j.ejrad.2021.109673 (2021).
Coroller, T. P. et al. Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS ONE 12, e0187908. https://doi.org/10.1371/journal.pone.0187908 (2017).
Park, Y. W. et al. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur. Radiol. 29, 4068–4076. https://doi.org/10.1007/s00330-018-5830-3 (2019).
Ke, C. et al. Differentiation between benign and nonbenign meningiomas by using texture analysis from multiparametric MRI. J. Magn. Reson. Imaging 51, 1810–1820. https://doi.org/10.1002/jmri.26976 (2020).
Morin, O. et al. Integrated models incorporating radiologic and radiomic features predict meningioma grade, local failure, and overall survival. Neurooncol. Adv. 1, vdz011. https://doi.org/10.1093/noajnl/vdz011 (2019).
Zhu, Y. et al. A deep learning radiomics model for preoperative grading in meningioma. Eur. J. Radiol. 116, 128–134. https://doi.org/10.1016/j.ejrad.2019.04.022 (2019).
Park, J. E., Park, S. Y., Kim, H. J. & Kim, H. S. Reproducibility and generalizability in radiomics modeling: Possible strategies in radiologic and statistical perspectives. Korean J. Radiol. 20, 1124–1137. https://doi.org/10.3348/kjr.2018.0070 (2019).
Boxerman, J. L. et al. Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro Oncol. 22, 1262–1275. https://doi.org/10.1093/neuonc/noaa141 (2020).
Kaufmann, T. J. et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro Oncol 22, 757–772. https://doi.org/10.1093/neuonc/noaa030 (2020).
Zhu, J.-Y., Park, T., Isola, P. & Efros, A. A. In: Proceedings of the IEEE international conference on computer vision. 2223–2232.
Shaban, M. T., Baur, C., Navab, N. & Albarqouni, S. Staingan: Stain style transfer for digital histological images. 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), 953–956 (2019).
Shin, S. J. et al. Style transfer strategy for develo** a generalizable deep learning application in digital pathology. Comput. Methods Programs Biomed. 198, 105815. https://doi.org/10.1016/j.cmpb.2020.105815 (2021).
Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 131, 803–820. https://doi.org/10.1007/s00401-016-1545-1 (2016).
Avants, B. B., Tustison, N. & Song, G. Advanced normalization tools (ANTS). Insight J. 2, 1–35 (2009).
Roy, S., Butman, J. A. & Pham, D. L. Robust skull strip** using multiple MR image contrasts insensitive to pathology. Neuroimage 146, 132–147. https://doi.org/10.1016/j.neuroimage.2016.11.017 (2017).
Fonov, V. et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 54, 313–327. https://doi.org/10.1016/j.neuroimage.2010.07.033 (2011).
van Griethuysen, J. J. M. et al. Computational radiomics system to decode the radiographic phenotype. Can. Res. 77, e104–e107. https://doi.org/10.1158/0008-5472.Can-17-0339 (2017).
Zwanenburg, A., Leger, S., Vallières, M. & Löck, S. Image biomarker standardisation initiative. ar**v preprint https://arxiv.org/abs/1612.07003 (2016).
Lusa, L. Improved shrunken centroid classifiers for high-dimensional class-imbalanced data. BMC Bioinformatics 14, 1–13 (2013).
Isola, P., Zhu, J.-Y., Zhou, T. & Efros, A. A. in Proceedings of the IEEE conference on computer vision and pattern recognition. 1125–1134.
Wu, H., Zheng, S., Zhang, J. & Huang, K. In: Proceedings of the 27th ACM international conference on multimedia. 2487–2495.
Russell, S. & Norvig, P. Artificial intelligence: A modern approach. (2002).
Heusel, M., Ramsauer, H., Unterthiner, T., Nessler, B. & Hochreiter, S. Gans trained by a two time-scale update rule converge to a local nash equilibrium. ar**v preprint https://arxiv.org/abs/1706.08500 (2017).
Salimans, T. et al. Improved techniques for training gans. ar**v preprint https://arxiv.org/abs/1606.03498 (2016).
Van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9 (2008).
Liu, M.-Y., Breuel, T. & Kautz, J. Unsupervised image-to-image translation networks. ar**v preprint https://arxiv.org/abs/1703.00848 (2017).
Lee, D., Moon, W.-J. & Ye, J. C. Assessing the importance of magnetic resonance contrasts using collaborative generative adversarial networks. Nat. Mach. Intell. 2, 34–42 (2020).
Sharma, A. & Hamarneh, G. Missing MRI pulse sequence synthesis using multi-modal generative adversarial network. IEEE Trans. Med. Imaging 39, 1170–1183 (2019).
Conte, G. M. et al. Generative adversarial networks to synthesize missing T1 and FLAIR MRI sequences for use in a multisequence brain tumor segmentation model. Radiology 299, 203786 (2021).
Chen, Y. et al. in 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018). 739–742 (IEEE).
Sounderajah, V. et al. Develo** specific reporting guidelines for diagnostic accuracy studies assessing AI interventions: The STARD-AI Steering Group. Nat Med 26, 807–808. https://doi.org/10.1038/s41591-020-0941-1 (2020).
Marcadent, S. et al. Generative adversarial networks improve the reproducibility and discriminative power of radiomic features. Radiology: Artificial Intelligence 2, e190035 (2020).
Bangalore Yogananda, C. G. et al. A novel fully automated MRI-based deep-learning method for classification of IDH mutation status in brain gliomas. Neuro Oncol. 22, 402–411. https://doi.org/10.1093/neuonc/noz199 (2020).
Hamerla, G. et al. Comparison of machine learning classifiers for differentiation of grade 1 from higher gradings in meningioma: A multicenter radiomics study. Magn. Reson. Imaging 63, 244–249. https://doi.org/10.1016/j.mri.2019.08.011 (2019).
Hagiwara, A., Fujita, S., Ohno, Y. & Aoki, S. Variability and standardization of quantitative imaging: Monoparametric to multiparametric quantification, radiomics, and artificial intelligence. Invest Radiol. 55, 601–616. https://doi.org/10.1097/rli.0000000000000666 (2020).
Borji, A. Pros and cons of gan evaluation measures. Comput. Vis. Image Underst. 179, 41–65 (2019).
Acknowledgements
This research received funding from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, Information and Communication Technologies & Future Planning (2020R1A2C1003886). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2020R1I1A1A01071648). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HI21C1161), and by a Severance Hospital Research fund for Clinical excellence (C-2021-0019). This research was also funded by the Bio Industrial Strategic Technology Development Program (20003883, 20005021) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health &Welfare, Republic of Korea (HR16C0001).
Author information
Authors and Affiliations
Contributions
S.C.Y. and S.S.A. designed and conceptualized the study. Y.W.P. and S.M.L. collected the data. J.E. and H.L. performed machine learning analysis.Y.W.P. and S.J.S. wrote the main manuscript text. S.C.Y. and S.S.A. revised the manuscript. All authors reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, Y.W., Shin, S.J., Eom, J. et al. Cycle-consistent adversarial networks improves generalizability of radiomics model in grading meningiomas on external validation. Sci Rep 12, 7042 (2022). https://doi.org/10.1038/s41598-022-10956-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10956-9
- Springer Nature Limited
This article is cited by
-
Multicenter PET image harmonization using generative adversarial networks
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Intelligent noninvasive meningioma grading with a fully automatic segmentation using interpretable multiparametric deep learning
European Radiology (2023)
-
Preoperative surgical risk assessment of meningiomas: a narrative review based on MRI radiomics
Neurosurgical Review (2022)