Abstract
Nd2Fe14B and Nd2−xDyxFe14B (x = 0.25, 0.50) particles were prepared by the modified co-precipitation followed by reduction–diffusion process. Bright field scanning transmission electron microscope (BF-STEM) image revealed the formation of Nd–Fe–B trigonal prisms in [− 101] viewing zone axis, confirming the formation of Nd2Fe14B/Nd2−xDyxFe14B. Accurate site for the Dy substitution in Nd2Fe14B crystal structure was determined as “f” site by using high-angle annular dark field scanning transmission electron microscope (HAADF-STEM). It was found that all the “g” sites are occupied by the Nd, meanwhile Dy occupied only the “f” site. Anti-ferromagnetic coupling at “f” site decreased the magnetic moment values for Nd1.75Dy0.25Fe14B (23.48 μB) and Nd1.5Dy0.5Fe14B (21.03 μB) as compared to Nd2Fe14B (25.50 μB). Reduction of magnetic moment increased the squareness ratio, coercivity and energy product. Analysis of magnetic anisotropy at constant magnetic field confirmed that “f” site substitution did not change the patterns of the anisotropy. Furthermore, magnetic moment of Nd2Fe14B, Nd2−xDyxFe14B, Nd (“f” site), Nd (“g” site) and Dy (“f” site) was recorded for all angles between 0° and 180°.
Similar content being viewed by others
Introduction
Nd2Fe14B type magnets have the highest recorded maximum energy product (BH)max among permanent magnets1,2,3,4,5. They have drawn huge attention due to their wide applications in wind turbines, hybrid-electric vehicles, transducers, magnetic fluids, magnetic elastomers, sensors, magnetic separators, magnetic levitation systems, loudspeakers, generators and motors6,7,8,9,10,11. Current maximum coercivity of unsubstituted Nd2Fe14B is recorded as 10 kOe, which makes it economically inadequate for commercial purpose. However, magnetic properties of Nd2Fe14B have been improved significantly by the substitution of Nd with rare earth elements (RE)12,13,14,15,16,17,18,19,20,21. Hence the synthesis of Nd2−xRExFe14B by RE substitution for Nd is vital for the applications of Nd2Fe14B on commercial scale. The site of RE atoms in the Nd2−xRExFe14B crystal lattice is very critical for its anisotropy and magnetic moment. Nd2Fe14B is a tetragonal structure with four formula units and 68 atoms, which has two unequal sites for Nd (RE), “g” site and “f” site. When RE substitutes for Nd in Nd2Fe14B crystal lattice, it may either substitute at “f” or “g” site. Aftab, Kitagawa and Liu et al. have worked to estimate the substitution site for RE in Nd2Fe14B theoretically, by using density function theory (DFT)19,20,21. Aftab et al. applied Vienna ab initio simulation package (VASP) with a pseudo-potential and projected-augmented wave (PAW) method. Kitagawa et al. applied the linear combination of pseudo-atomic-orbital (LCPAO) method, while Liu et al. used full potential plane-wave plus muffin-tin orbital method.
Experimental evidences for Dy site in (Nd-Dy)2Fe14B crystal lattice was provided by Itakura et al.22 Although they presented STEM-HAADF image but d-spacing value for the [001] facet deviated from the standard values. Absence of the stoichiometric ratio between Nd:Dy:Fe and distribution of Dy only near grain boundary raised more questions, hence it was interesting for the contemporary researchers to answer them. Saito et al.23 used neutron diffraction technique and determined the quantitative distribution of Dy at substitution sites. They postulated that Dy substitutes at both the “f” and “g” sites, and population of Dy on each site depends on the annealing temperature of the experiment. This finding was different from the previous studies, those suggested that Dy only substitutes at “f” site. Furthermore, magnetic anisotropic properties of “f” and “g” sites in Nd2−xDyxFe14B were not yet studied. In order to answer the questions discussed above and a comprehensive study of “f” and “g” sites in Nd2−xDyxFe14B was required.
In this work, we prepared Nd2Fe14B and Nd2−xDyxFe14B with optimized co-precipitation method followed by reduction–diffusion (R–D) process. This co-precipitation method is similar with the approach reported by Ma and Palaka et al. with minor modification24,25. Detail of this modification is explained in the “supplementary information”. Site preference of Dy in Nd1.5RE0.5Fe14B particles was confirmed through the crystal structure determination by using HAADF-STEM. Effect of Dy substitution on the magnetic moment, magneto-crystalline anisotropy energy and coercivity of the Nd2−xDyxFe14B particles is also studied. Nd2−xDyxFe14B sample was rotated in the rotating angle range of 0° to 180° at the constant magnetic field to observe variation of anisotropic patterns after the substitution. Effect of the substitution on the anisotropic properties of “f” and “g” sites in the Nd2−xDyxFe14B crystal is also studied.
Methods
Materials
All chemicals used in this work, including neodymium (III) chloride hexahydrate (NdCl3·6H2O), iron (III) chloride hexahydrate (FeCl3·6H2O), sodium hydroxide (NaOH), boric acid (H3BO3), calcium hydride (CaH2), dysprosium (III) chloride hexahydrate (DyCl3·6H2O), ethyl alcohol (C2H5OH) and acetone (CH3COCH3) were analytical grade and obtained from Sigma-Aldrich Co.
Preparation of Nd1.5RE0.5Fe14B particles
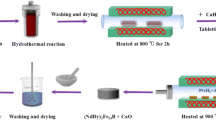
Schematics for the preparation procedure is shown as the Fig. S1 and detailed process of synthesis is explained in the “supplementary information”. In brief, all the metal chlorides of Nd, Fe and Dy were dissolved in deionized (D.I.) water under the stirring to obtain the clear solution. NaOH (3.5 M) was added to the solution in a drop wise until the pH approached to 13. The resultant solution was stirred continuously for 4 h. Then, products were washed twice with D.I. water and ethanol, and dried overnight at 353 K (80 °C) followed by annealing at 973.15 K (700 °C) for 30 min to convert all hydroxides to oxides. The product was mixed with boric acid and CaH2 in a glove box and then pressed into pellet form. The pellet was undergone to R–D via annealing at 1273.15 K (1000 °C) for 3 h with Ar flowing in the furnace. The pellet after R–D was pulverized and washed with water to remove calcium oxide (CaO) completely and rinsed with acetone. To obtain Nd2Fe14B, weight ratio of Nd:Fe:B was kept as 15:77:8, as per standard ratio for the synthesis of Nd2Fe14B by R–D. In order to produce Nd1.75Dy0.25Fe14B and Nd1.5RE0.5Fe14B, Nd:Dy:Fe:B ratio was kept as 13.12:1.88:77:8 and 11.25:3.75:77:8, hence 12.5% and 25% of Nd was substituted with Dy, respectively. Nd:Dy ratios in Nd1.75Dy0.25Fe14B and Nd1.5Dy0.5Fe14B were determined as 7.17:1 and 3.13:1 experimentally (Figs. S3, S4). These values were quite close to the theoretically expected ratios of Nd:Dy ratios, 7:1 and 3:1.
Samples preparation for SEM and TEM
Nd2Fe14B and Nd2−xDyxFe14B powder samples were placed on the movable lower ram and then the prepared solder pieces were placed on the sample. Electrically conductive polymer was poured to encapsulate the solder and powder samples, then heated at 180 °C for 6 min and then pressed under the pressure of 30 kN. Prepared sample was mounted on SEM holder, then mechanically polished with SiC paper, diamond suspension and colloidal silica, subsequently characterized with FE-SEM.
Specimens for TEM (transmission electron microscope) were prepared by focused ion beam (FIB-NX2000, Hitachi) using the lift-out technique. For TEM measurement, sample was treated as the same process reported by Kim et al.26 and orientation of sample along [100] zone axis was confirmed by using electron backscatter diffraction (EBSD) (TEAM™ Pegasus, Ametek Co. Ltd. USA).
Characterization
Crystal structure and phases were determined by X-ray diffraction (XRD) patterns using a Rigaku Diffractometer (XRD, Rigaku). The morphology, size and elemental distribution were observed with field emission scanning electron microscope (JSM-7000F, JEOL), conventional transmission electron microscopy (TEM, JEM-2100F) and aberration corrected TEM (ARM-200F) with energy dispersive X-ray spectroscopy (EDS). TEM was operated at the accelerating voltage of 200 kV. Angular dependent magnetic properties were measured by magnetic property measurement system (MPMS3-Evercool) equipped with rotator. Magnetic properties (M-H curves) of final product were measured by Physical Property Measurement System (PPMS, Evercool II–9 T) in the vibrating sample magnetometer mode. Magnetic field in the range of 7179.3 to − 7179 kA/m was applied to measure the magnetic properties of the final products. JEOL JEM-ARM200F Cs-corrected TEM was used to obtain HAADF-STEM images.
Numerical method
Full potential linearized augmented plane wave method, as implemented in the Wien2k code, was used to calculate the magnetic moment of Nd2Fe14B and Nd2−xDyxFe14B. Details are described in “supplementary information”.
Results and discussion
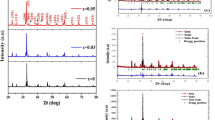
Nd2Fe14B and Nd2−xDyxFe14B magnetic particle were prepared by co-precipitation followed by reduction diffusion process. Chemical reactions and mechanisms during co-precipitation and R–D processes are explained in the “supporting information”. R–D reaction follows the mechanism proposed by the Haider et al.27 XRD patterns for Nd2Fe14B and Nd2−xDyxFe14B particles are similar due to the almost same crystal structure (Fig. 1a). They have the Nd2Fe14B (JCPDS #36-1296) as main phase with additional peaks corresponding to the extra Nd phase. Nd substitution with Dy makes the peaks position be shifted to the right side (Fig. 1a).
(a) XRD patterns of Nd2Fe14B and Nd2−xDyxFe14B particles (b) crystal parameters “a” and “c” of Nd2Fe14B and Nd2−xDyxFe14B. BSE-SEM images of (c) Nd2Fe14B (d) Nd1.75Dy0.25Fe14B and (e) Nd1.5Dy0.5Fe14B particles. Particle size distribution of (f) Nd2Fe14B (g) Nd1.75Dy0.25Fe14B and (h) Nd1.5Dy0.5Fe14B particles.
This is due to the different crystal lattice parameters of Nd2−xDyxFe14B and to the smaller ionic radii of Dy (178 pm) as compared to the Nd (181 pm). Both “a “and “c” dimensions of the crystal lattice were decreased after the Dy substitution in the Nd2Fe14B crystal lattice (Fig. 1b). The decreased values of these Nd1.5RE0.5Fe14B crystal parameters are also evidence of Dy substitution in Nd2Fe14B crystal. In order to calculate the lattice parameters (“a” and “c”), at first d-spacing values were calculated from the XRD patterns (Fig. 1). h, k and l values were determined from Nd2Fe14B JCPDS #36-1296. Finally, “c” and “a” value were calculated by the following equation as the same method reported by Rahimi17, Cullity28, and Charbel29, et al.
SEM-BSE images in Fig. 1c–e revealed that the particles were in irregular shape and the size distribution is in the range of 0.3–10 μm. The Nd2Fe14B had the largest average particle size as 3.5 μm while Nd1.5Dy0.5Fe14B had the least average particle size as 0.8 μm. Nd1.75Dy0.25Fe14B had the average particle size of 1.7 μm. SEM–EDS confirmed that Nd and Dy are homogeneously distributed with Fe in the particles (Figs. S5, S6). The microstructure, elemental composition and distribution of Nd1.5Dy0.5Fe14B particles were evaluated with STEM as shown in Fig. 2. The elemental distribution of Nd, Dy, Fe, and O was investigated by using STEM-EDS, which confirmed that the Dy was substituted for Nd in the crystal structure and it was distributed inside the grain. Figure 2b is the line EDS taken from the blue circle of LAADF-STEM image of Nd1.5Dy0.5Fe14B. Figure 2c shows the EDS line profile of interface between two fused Nd1.5Dy0.5Fe14B particles, as marked with the blue circle in Fig. 2a. No oxygen was detected in EDS map** because boundaries of the particles were not exposed to the water during washing process.
To evaluate the crystallinity of the specimen, SAED patterns (Fig. 3b) of the marked area with red circle in the TEM image (Fig. 3a) were obtained. It was confirmed that the particles produced were single crystalline. It was deduced by SADP of strong diffraction maxima that each grain was completely single crystalline. Figure 3d shows the high resolution BF-STEM image of the Nd1.5Dy0.5Fe14B observed at the [− 101] zone axis and Fig. 3e represents the corresponding atomic arrangement simulated by JEMS software (P. Stadelmann, www.jems-saas.ch).
Both the BF-STEM image and simulated atomic arrangement showed the series of trigonal prism units. Figure 3c illustrates the arrangement of the atoms in the prism. Boron atom occupies the center of trigonal prisms surrounded with three nearest Fe atoms on top and the three Fe atoms at bottom. The triangular prism facets participate to form the complete tetragonal Nd2Fe14B lattice.
To know the accurate site of the RE in the crystal lattice, HAADF-STEM image was taken at the [100] zone axis as shown in Fig. 4. At [100] zone axis, columns having “4f” site and “4 g” site of Nd/Dy can be clearly distinguished. In addition, there is no Fe atom overlap** at each Nd position because of different locations of Fe and RE at [100] zone axis. Peak intensity of the histogram increases with the average atomic number, the HAADF-STEM image can be used to distinguish the Dy and Nd, and their positions (“f” or “g” site). Figure 4b is HAADF-STEM image, which confirms the same arrangement of atoms as the standard Nd2Fe14B [100] zone axis (Fig. 3a). Intensity histogram for red dotted panel in Fig. 4b was acquired. It is observed that the intensity of atoms (Dy) at “4f” column is higher than that of the atom (Nd) at “4 g” column. Higher peak intensity confirms that the substitution site of Dy is “4f” site because the atomic number of Dy (66) is larger than that of Nd (60), which leads to the higher intensity as compared to the Nd. The ‘a’ value of Nd1.5Dy0.5Fe14B crystal lattice is 8.78 Å (Fig. 3c), which is well consistent with the standard Nd1.5Dy0.5Fe14B value.
Standard Nd2Fe14B have ‘a’ and ‘b’ values of lattice parameters as 8.80 Å30. Standard distance of atomic column between “4f” site and “4 g” site of Nd2Fe14B is 1.1 Å. In this study, the obtained distance is 1.09 Å as shown in histogram Fig. 4c. This is well matched with theoretical value. A slight error is due to a noise induced by the fine drift of the sample or the poisson noise in the STEM. Hence, site preference for the Dy in Nd2Fe14B is proved to be “4f” as the previously theoretically reported by Liu et al.21. It was found that 100% “g” sites are occupied by the Nd (Fig. 5) and Dy was substituted only at “f” site.
Fe is ferromagnetic with electronic configuration of [Ar] 3d6 4s2. This electronic configuration shows that it has eight valance electrons. Arrangement of the electrons in the relevant orbitals is shown in the rigid band model as Fig. 6c. Density of the electrons was taken on X-axis and energy was taken on Y-axis. Ef indicates the Fermi level of the rigid band. Energy level of 3-d electrons is similar to the 4 s electrons, hence, there is no movement of electrons between the 4s and 3d orbital. Four unpaired electrons will be in the spin up configuration. Presence of the unpaired electrons makes the Fe ferromagnetic.
(a) M–H curves, Mr, Ms, Magnetic moment, Hc, energy density and squareness ratio of Nd2Fe14B, Nd1.75Dy0.25Fe14B and Nd1.5Dy0.5Fe14B particles. (b) Schematic illustration to explain the ferro and anti-ferromagnetic coupling between Nd, Dy and Fe (c) Explanation of coupling and hybridization between Nd, Dy and Fe.
Being completely filled, 5d orbitals in Nd or Dy do not play any role to determine the magnetic properties of Nd or Dy. However, Nd and Dy have unpaired electrons in the 4f orbitals, those impart the ferromagnetic character. Orbital magnetic moment (L) direction of the unpaired valance electrons in Dy is opposite to the Nd because 4f electrons in Dy are in spin down state. Nd has the unpaired electrons in the spin up direction, hence, they are ferromagnetic with the Fe.
Simultaneously Dy with the spin down configuration couples anti-ferromagnetically with Fe and Nd (Fig. 6c). 3d band in Fe may hybridize with the 5d orbital of the neighboring Nd and/or Dy. Schematic illustration of the exchange coupling and hybridization in the Nd2Fe14B and Nd2−XDyXFe14B is shown in Fig. 6c.
Magnetic moment of Nd2−xDyxFe14B have been strongly affected by Dy owing to its anti-ferromagnetic coupling with Fe and Nd. Individual values of magnetic moments of Nd2Fe14B, Nd1.75Dy0.25Fe14B and Nd1.5Dy0.50Fe14B, were determined as 25.50, 23.48, and 21.03 μB, respectively. Magnetic moments were determined by the the Ms values from M–H curves (Fig. 6a). Complete M-H curves with applied magnetic field range of − 9.5 to 9.5 T are provided in supplementary information as Fig. S-7. These experimentally determined values of magnetic moment are lower than the values by theoretical calculation because the theoretical parameter conditions are not fixed as the experimental ones21. For example, the theoretical calculation was based on the temperature at 5 K and all particles are single domain and un-oxidized. Dy coupled anti-ferromagnetically to Fe in the crystal lattice, resulting in the lower magnetic moment of Nd2−xDyxFe14B. The change of magnetic moment critically affected the coercivity. From the Fig. 6 the coercivity (Hc) values of Nd2Fe14B, Nd1.75Dy0.25Fe14B and Nd1.5Dy0.50Fe14B were determined as 4.58, 5.84 and 9.55 kOe, respectively. Nd2−xDyxFe14B showed the higher Hc due to stronger anisotropy energy and reduced magnetic moment. Additionally, Dy substituted particles have a smaller grain size as shown in SEM results. It is well known that coercivity increases as particle size gets smaller and approaches to the single domain size. The increasing order of coercivity as Nd2Fe14B < Nd1.75Dy0.25Fe14B < Nd1.5Dy0.5Fe14B and the decreasing order of magnetic moment as Nd2Fe14B > Nd1.75Dy0.25Fe14B > Nd1.5Dy0.5Fe14B were obtained from M–H curves.
Energy density or energy product is the amount of energy stored in the anisotropic Nd2Fe14B (or Nd2−xDyxFe14B) lattice because of arrangement of the atoms in the crystal. It is confirmed that the Dy substitutions results in the higher energy density. Energy densities for Nd2Fe14B, Nd1.75Dy0.25Fe14B and Nd1.5Dy0.50Fe14B were recorded as 39.71, 50.29 and 53.71 kJ/m3, respectively. Mr (emu/g), Ms (emu/g), squareness ratio (Sq), magnetic moment (μB), coercivity (Hc), and energy density values for the all Nd2Fe14B and Nd2−xDyxFe14B particles are shown in Fig. 6b, comparatively. M-H curve with S.I. units of coercivity (kA/m) is provided in supplementary information as Fig. S8.
Nd2Fe14B and Nd2−xDyxFe14B are expected to be anisotropic, hence, magnetic moment and energy density are angular dependent magnetic properties. Closely packed particles of Nd2Fe14B and Nd2−xDyxFe14B were aligned at 5 T with easy direction of magnetization, then magnetic field was reduced to the 200 Oe. Thereafter, sample was rotated in the angle range of 0°–180°. Figure 7a explains the preparation of the sample for the measurement of magnetic anisotropy. During the measurement at MPMS, the magnetic particles were closely packed, which stopped the rotation of the particles at low applied magnetic field (200 Oe). All processes were performed at room temperature so that the effect of thermal energy was neglected.
(a) Sample preparation for the measurement of magnetic anisotropy (b) Magnetic moment of Nd2Fe14B, Nd1.75Dy0.25Fe14B and Nd1.5Dy0.5Fe14B particles as a function of rotation angle. Magnetic moment at the “f” and “g” sites in (c) Nd2Fe14B (d) Nd1.5Dy0.5Fe14B (e) Nd1.75Dy0.25Fe14B as a function of rotation angle.
Figure 7b shows the anisotropic character of the Nd2Fe14B and Nd2−xDyxFe14B. When magnetic particles were rotated in the range of 0°–180° at constant applied magnetic field of 200 Oe, magnetic moment of the particles was changed significantly. Nd2Fe14B and Nd2−xDyxFe14B have maximum magnetic moment along “c” crystal direction, with parallel/anti-parallel orientation to the applied field of (θ = 0°, 180°). This is because of the “c” crystal dimension is easy direction for magnetization. On the contrary, along “a and b” crystal direction magnetic moment approached to zero.
Maximum magnetic moment value (24.13 μB) of Nd2Fe14B obtained during rotation was lower than the magnetic moment values obtained from M–H curves (25.50 μB). Reduced value of magnetic moment was observed because of weak applied magnetic field (200 Oe) during the rotation of the magnetic particles. However, the trend of magnetic moment variation for both the Nd2Fe14B and Nd2−xDyxFe14B was same. Conclusively, substitution of Dy for Nd does not affect the anisotropic patterns of the crystal, however, value of the energy density can be changed. Figure S-8 explains the interaction of the applied magnetic field and the electron spin density of Nd2Fe14B during the rotation.
Magnetic moments of Nd2Fe14B, Nd2−xDyxFe14B and Nd were calculated theoretically by full potential linearized augmented plane wave method, as implemented in the Wien2k code. Individual magnetic moments of Nd and Dy atoms at different sites were also calculated theoretically by this method. Theoretically calculated magnetic moment values for Nd2Fe14B, Nd (f) and Nd (g) were 30.20, 3.32 3.30 μB, respectively. Experimentally determined values for the Nd2Fe14B, Nd (f) Nd (g) were 24.3, 2.69, 2.67 μB, respectively. Details of experimental and theoretical calculations are provided in the “supplementary information”.
Theoretically Nd always occupies the “g” site in Nd2Fe14B, Nd1.75Dy0.25Fe14B and Nd1.5Dy0.50Fe14B19,20,21. This is also confirmed in this work (Fig. 4). Hence in case of Nd1.75Dy0.25Fe14B and Nd1.5Dy0.50Fe14B there is negligible change in the magnetic moment on the “g” site after substitution. In Nd2Fe14B formula unit, Nd is distributed equally among 50% “f” and 50% “g” sites. Magnetic moment on “f” and “g” sites of in Nd2Fe14B is determined as 2.69 and 2.67 μB, respectively.
In Nd1.75Dy0.25Fe14B and Nd1.5Dy0.50Fe14B, Nd occupies 75% and 50% “f” sites, simultaneously Dy occupies leftover 25% and 50% “f” sites respectively. Hence magnetic moment on one “f” sites in Nd1.75Dy0.25Fe14B and Nd1.5Dy0.50Fe14B was determined as 0.82 and − 1.05 μB, respectively. Dy, which has almost double magnetic moment (5.34 μB) as compared to the Nd (2.45 μB), reduces the magnetic moment very effectively after the substitution. Reduction of magnetic moment of in the Nd2−xDyxFe14B formula unit is actually the reduction of the magnetic moment of the “f” site.
After obtaining the experimental values of the magnetic moments of the Nd2Fe14B, Nd (f) and Nd (g), anisotropic behavior of “f” and “g” sites was studied. Figure 7a explains the sample preparation for the measurement of magnetic anisotropy. Nd2Fe14B, and Nd1.75Dy0.25Fe14B particles were compressed in the plastic discs and then attached to the rotating plate with the resin. Wires and springs used in the apparatus are made of Cu, which is non-magnetic. When these samples were kept in the MPMS they were rotated by application of force on the spring. Magnetic moment values of the “f” and “g” sites in Nd2Fe14B and Nd2−xDyxFe14B at various angles of rotation are given in the Table 1. Figure 7b graphically illustrates that the magnetic moment of Nd2Fe14B and Nd2−xDyxFe14B as the function of rotating angle in the constant magnetic field. Figure 7c–e shows the variation of individual magnetic moments of “f” and “g” sites as the function of rotating angle.
Conclusions
Nd2Fe14B and Nd2−xDyxFe14B (x = 0.25, 0.50) particles were successfully prepared by the modified co-precipitation method followed by reduction–diffusion process. Micro-structure analysis of the composition and distribution of elements confirmed the homogeneous distribution of Dy atoms in the crystal lattice of Nd2Fe14B and Dy substitution at “4-f” site. Orbital magnetic moment (L) direction of the unpaired valance electrons of Dy was opposite to the Fe and Nd which resulted in the anti-ferromagnetic coupling between them. Nd substitution with Dy on “f” site reduced the magnetic moment of Nd2DyxFe14B due to anti-ferromagnetic coupling with Nd and Fe, but enhanced the energy density, squareness ratio and coercivity. Furthermore, it was found that Nd2Fe14B and Nd2−xDyxFe14B particles have maximum magnetic moment when they are aligned parallel or anti-parallel to the applied magnetic field and have the minimum energy density when they are rotated perpendicular to the applied magnetic field. Conclusively, “f” site substitution of Nd with Dy in Nd2Fe14B did not change the anisotropic patterns of Nd2−xDyxFe14B.
References
Muljadia, M. & Sardjonoa, P. Preparation and characterization of 5 wt% epoxy resin bonded magnet NdFeB for micro generator application. Supraped. Ener. Proced. 68, 282–287. https://doi.org/10.1016/j.egypro.2015.03.257 (2015).
Honshima, M. & Ohashi, K. High-energy NdFeB agnets and their applications. J. Mat. Engg. Perform. 3(2), 218–222. https://doi.org/10.1007/BF02645846 (1994).
Ma, X. H. et al. Preparation of Nd–Fe–B by nitrate–citrate auto-combustion followed by the reduction–diffusion process. Nanoscale 7, 8016–8022. https://doi.org/10.1039/C5NR01195G (2015).
Jeong, H. et al. Chemical synthesis of Nd2Fe14B hard phase magnetic nanoparticles with an enhanced coercivity value: Effect of CaH2 amount on the magnetic properties. N. J. Chem. 40, 10181. https://doi.org/10.1039/C6NJ02436J (2016).
Kim, C. W., Kim, Y. H., Pal, U. & Kang, Y. S. Facile synthesis and magnetic phase transformation of Nd–Fe–B nanoclusters by oxygen bridging. J. Mater. Chem. C 1, 27. https://doi.org/10.1039/C2TC00083K (2013).
Amirouche, F., Zhou, Y. & Johnson, T. Current micro pump technologies and their biomedical applications. Microsyst. Technol. 15, 647–666. https://doi.org/10.1007/s00542-009-0804-7 (2009).
Chen, Z., Miller, D. & Herchenroeder, J. High performance nanostructured Nd–Fe–B fine powder prepared by melt spinning and jet milling. J. Appl. Phys. 107, 09A730.1-0A7303.4. https://doi.org/10.1063/1.3348544 (2010).
Riaño, S. & Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 17, 2931–2942. https://doi.org/10.1039/C5GC00230C (2015).
Reimer, M. V., Schenk-Mathes, H. Y., Hoffmann, M. F. & Elwert, F. Recycling decisions in 2020, 2030, and 2040, when can substantial NdFeB extraction be expected in the EU?. Metals 8, 867.1–867.5. https://doi.org/10.3390/met8110867 (2018).
Cha, H. G., Kim, Y. H., Kim, C. W. & Kang, Y. S. Preparation for exchange-coupled permanent magnetic composite between α-Fe (soft) and Nd2Fe14B (hard). Curr. Appl. Phys. 7(4), 400–403. https://doi.org/10.1016/j.cap.2006.09.010 (2007).
Akiya, T., Kato, H., Sagawa, M. & Koyama, K. Enhancement of coercivity in Al and Cu added Nd-Fe-B sintered magnets by high field annealing. IOP Conf. Series Mat. Sci. Eng. 012034(1–012034), 7. https://doi.org/10.1088/1757-8981/1/1/012034 (2009).
Yu, N. J., Pan, N. J., Zhang, P. Y. & Ge, H. L. The origin of coercivity enhancement of sintered NdFeB magnets prepared by Dy addition. J. Magnet. 18(3), 235–239. https://doi.org/10.4283/JMAG.2013.18.3.235 (2013).
Li, W. F., Sepehri-Amin, H., Ohkubo, T., Hase, N. & Hono, K. Distribution of Dy in high-coercivity (Nd, Dy)–Fe–B sintered magnet. Acta Mater. 59, 3061–3069. https://doi.org/10.1016/j.actamat.2011.01.046 (2011).
Tan, X., Parmar, X., Zhong, Y., Chaudhary, V. & Ramanujan, R. V. Effect of Dy substitution on the microstructure and magnetic properties of high (BH)max Nd-Dy-Fe-Co-B nanoparticles prepared by microwave processing. J. Magn. Magn. Mater. 471, 278–285. https://doi.org/10.1016/j.jmmm.2018.09.017 (2019).
Wenlong, Y. et al. Influence of gadolinium on microstructure and magnetic properties of sintered NdGdFeB magnets. J. Rare Earth. 30, 133–136. https://doi.org/10.1016/S1002-0721(12)60009-X (2012).
Zhong, Y., Chaudhary, V., Tan, X., Parmar, H. & Ramanujan, R. V. High coercivity Dy substituted Nd-Fe-Co-B magnetic nanoparticles produced by mechanochemical processing. Magn. Magn. Mater. 475, 554–562. https://doi.org/10.1016/j.jmmm.2018.08.061 (2019).
Rahimi, H., Ghasemi, A., Mozaffarinia, R. & Tavoosi, M. Coercivity enhancement mechanism in Dy-substituted Nd–Fe–B nanoparticles synthesized by sol–gel base method followed by a reduction diffusion process. J. Magn. Magn. Mater. 429, 182–191. https://doi.org/10.1016/j.jmmm.2017.01.041 (2017).
Khan, I. & Hong, J. Electronic structure and magnetic properties of Nd2Fe14B. J. Kor. Phys. Soc. 68, 1409–1414. https://doi.org/10.3938/jkps.68.1409 (2016).
Alam, A., Khan, M., McCallum, R. W. & Johnson, D. D. Site-preference and valency for rare-earth sites in (R-Ce)2Fe14B magnets. Appl. Phys. Let. 102, 042402.1–042402.5. https://doi.org/10.1063/1.4789527 (2013).
Kitagawa, I. & Asari, Y. Magnetic anisotropy of R2Fe14B (R = Nd, Gd, Y): Density functional calculation by using the linear combination of pseudo-atomic-orbital method. Phys. Rev. B. 81, 214408.1–214408.7. https://doi.org/10.1103/PhysRevB.81.214408 (2010).
Liu, X. B. & Altounian, Z. The partitioning of Dy and Tb in NdFeB magnets: A first-principles study. J. Appl. Phys. 111, 07A701.1–07A701.3. https://doi.org/10.1063/1.3670054 (2012).
Itakura, M., Watanabe, N., Nishida, M., Daio, T. & Matsumura, S. Atomic-resolution X-ray energy-dispersive spectroscopy chemical map** of substitutional Dy atoms in a high-coercivity neodymium magnet. Jap. J. App. Phys. 52, 050201. https://doi.org/10.7567/JJAP.52.050201 (2013).
Saito, K., Doi, S., Abe, T. & Ono, K. Quantitative evaluation of site preference in Dy-substituted Nd2Fe14B. J. Alloy. Comd. 721, 476–481. https://doi.org/10.1016/j.jallcom.2017.04.155 (2017).
Ma, Z., Zhang, T. & Jiang, C. A facile synthesis of high performance SmCo5 nanoparticles. Chem. Eng. J. 264, 610–616. https://doi.org/10.1016/j.cej.2014.11.138 (2015).
Palaka, S. et al. A facile chemical synthesis of PrCo5 particles with high performance. J. Alloy. Comd. 812, 151674. https://doi.org/10.1016/j.jallcom.2019.151674 (2020).
Kim, T. A., Kang, M. C., Jung, G. B., Kim, D. S. & Yang, C. W. Novel method for preparing transmission electron microscopy samples of micrometer-sized powder particles by using focused ion beam. Microsc. Microanal. 23, 1055–1060. https://doi.org/10.1017/S1431927617012557 (2017).
Syed, K. H., **-Young, L., Dongsoo, K. & Kang, Y. S. Eco-friendly facile three-step recycling method of (Nd-RE)2Fe14B magnet sludge and enhancement of (BH)max by ball milling in ethanol. ACS Sustain. Chem. Eng. 8, 8156–8163. https://doi.org/10.1021/acssuschemeng.0c00584 (2020).
Cullity, B. D. Elements of X-ray Diffraction 340–341 (Addison-Wesley, 1956).
Charbel, T. Crystal structure, X-ray diffraction and Oblique geometry: Volumes, distances and angles. Eur. J. Phys. 2, 1–10 (2019).
Herbst, J. F., Croat, J. F., Pinkerton, F. E. & Yelon, W. B. Relationships between crystal structure and magnetic properties in Nd2Fe14B. Phys. Rev. B. 29, 1–4. https://doi.org/10.1103/PhysRevB.29.4176 (1984).
Acknowledgements
This work was supported by a National Research Council of Science and Technology (NST) grant by the Korea government (MSIT) (No. CRC-15-06-KIGAM). Y.S. Kang also appreciate for the financial support by the Leader Project at the Sogang University funded by the Ministry of Science and ICT through the National Research Foundation of Korea (No. 2020R1A3B3079715).
Author information
Authors and Affiliations
Contributions
S.K.H. performed experiment did characterization and wrote manuscript. Y.S.K. helped in data interpretation and manuscript writing. D.K. helped in experiment and characterization. C.-W.Y. and M.-C.K. did the characterization for structural analysis and helped in the data interpretation and figures making. J.H. provided simulation data for the magnetic moment.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haider, S.K., Kang, MC., Hong, J. et al. Determination of Dy substitution site in Nd2−xDyxFe14B by HAADF-STEM and illustration of magnetic anisotropy of “g” and “f” sites, before and after substitution. Sci Rep 11, 6347 (2021). https://doi.org/10.1038/s41598-021-85713-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85713-5
- Springer Nature Limited