Abstract
Evaluation of treatment response is among the major challenges in modern oncology. We herein used a monoclonal antibody targeting the EGF receptor (EGFR) labelled with the alpha emitter 213Bi (213Bi-anti-EGFR-MAb). EJ28Luc (bladder) and LN18 (glioma) cancer cells, both overexpressing EGFR, were incubated for 3 h with the radioimmunoconjugate. To assess the responses in the core carbon metabolism upon this treatment, these cancer cell lines were subsequently cultivated for 18 h in the presence of [U-13C6]glucose. 13C-enrichment and isotopologue profiles of key amino acids were monitored by gas chromatography–mass spectrometry (GC/MS), in order to monitor the impacts of the radionuclide-treatment upon glucose metabolism. In comparison to untreated controls, treatment of EJ28Luc cells with 213Bi-anti-EGFR-MAb resulted in a significantly decreased incorporation of 13C from [U-13C6]glucose into alanine, aspartate, glutamate, glycine, proline and serine. In sharp contrast, the same amino acids did not display less 13C-enrichments during treatment of the LN18 cells. The data indicate early treatment response of the bladder cancer cells, but not of the glioma cells though cell lines were killed following 213Bi-anti-EGFR-MAb treatment. The pilot study shows that the 13C-labelling approach is a valid tool to assess the responsiveness of cancer cells upon radionuclide-treatment in considerable metabolic detail.
Similar content being viewed by others
Introduction
Evaluation of response to treatment of cancerous diseases is among the major challenges in modern oncology. With [18F]FDG-PET, a clinically well-established imaging method is available, which helps to understand and to decipher therapeutic efficacy of anti-tumor therapies in vivo. This method relies on the fact that tumor cells show a high glycolytic turnover combined with the production of vast amounts of lactate, as discovered by Otto Warburg already in the last century1. However, as soon as the radioactive glucose analogue [18F]FDG gets trapped inside a cancer cell following internalization and enzymatic conversion by hexokinase II, no further metabolic steps can be investigated2. In contrast to the 18F-method, a labeling approach based on 13C-glucose could also glean downstream metabolic processes as monitored by gas chromatography–mass spectrometry (GC–MS) analysis of amino acids derived from glycolytic and citric acid (TCA) cycle intermediates3,4. Following a similar approach, metabolic heterogeneity in lung cancers was identified showing higher lactate metabolism compared to benign cells5,6,7. Beyond, pyruvate carboxylation was higher at the site of lung metastases compared to the primary tumor site in breast cancer cells8. These examples underline the interplay between different metabolic pathways and metastatic/oncological behavior. The approach using 13C-labeled glucose is therefore also a promising concept for the evaluation of targeted therapy, because early treatment responses following incubation of cancer cells with alpha-emitter immunoconjugates still need to be clarified.
In this study, we used an antibody targeting the epidermal growth factor receptor (EGRF) labelled with the alpha emitter 213Bi (213Bi-anti-EGFR-MAb), to explore treatment associated responses in two different cancer cell lines expressing EGRF. EGFR has been reported to be upregulated on the surface of tumour cells of glioblastoma, lung cancer, head and neck cancer, and bladder cancer. EGFR promotes tumour cell division and tumour growth and, due to its overexpression, it serves as a promising target for targeted therapy9,10,11,12.
In applying targeted alpha therapy (TAT) using the alpha-emitter 213Bi coupled to the anti-EGFR antibody matuzumab, we take advantage of the destructive potential of alpha-emitters as characterized by the high linear energy transfer of alpha-particles13, 14. As alpha emitters have only a short range in tissue, it is necessary to warrant a close delivery of the alpha emitter to the target cells. In our study, this is realized using an anti-EGFR-antibody. 213Bi-anti-EGFR-MAb selectively eradicates cancer cells that show EGFR overexpression15.
We previously showed that hyperpolarized 13C enriched pyruvic acid can be used to monitor treatment related changes of 213Bi-anti-EGFR-MAb15. However, the metabolic turnover of pyruvic acid to lactate is only one of many metabolic steps that occur in a cell, and experimental settings using hyperpolarized 13C enriched biomolecules cannot fully resolve metabolic pathways and fluxes in a cell or organ under study. Potential further complications of this approach arise due to unpredictable kinetic rates of metabolic enzymes in the complex environment of human cells and the relatively short half-life of hyperpolarized substrates.
In this study, we have monitored early metabolic response to treatment with 213Bi-anti-EGFR-MAb in two different cancer cell lines in vitro via GC–MS-based analysis of 13C-glucose incorporation into amino acids. We hypothesize that cells treated with an alpha emitter undergo massive changes of metabolism prior to apoptosis. To date, data on effects of alpha-irradiation upon metabolism of cancer cell is still sparse. Our aim was therefore to use the 13C-based metabolic pathway analysis monitored by GC–MS of amino acids to comprehensively determine the impact of an alpha emitter treatment upon the core metabolic pathways in cancer cells. To the best of our knowledge, this is the first study having used the 13C-technology to address this question.
Materials and methods
Cell lines
The human urothelial carcinoma cell line EJ28Luc, isolated from a primary bladder carcinoma, was grown in RPMI medium supplemented with 10% fetal calf serum and 1% non-essential amino acids (Biochrom, Berlin, Germany) in a humified atmosphere containing 5% CO2 at 37 °C. Transfection of cells was previously carried out with plasmid pcDNA3.1 containing the coding sequence of firefly (Photinus pyralis) luciferase under the control of the cytomegalovirus promoter12. The human glioma cell line LN18 was cultured in RPMI medium supplemented with 10% fetal calf serum. Cells were harvested after rinsing the monolayer with an EDTA/PBS solution (1 mM EDTA in PBS; Biochrom) for 10 min at 37 °C, respectively.
EJ28Luc cells were a gift from Birgit Pfost. LN18 cells were gifted from Jürgen Schlegels’ lab. All methods were carried out in accordance with relevant guidelines and regulations.
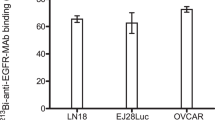
Both cell lines were chosen based on the high EGFR expression as deduced from binding of 213Bi-anti-EGFR-MAb > 60%, allowing for a targeted treatment. Binding of 213Bi-anti-EGFR to both cell lines was shown previously15.
Coupling of 213 Bi to an anti-EGFR-MAb
The anti-EGFR-MAb (matuzumab; Merck, Darmstadt, Germany) was conjugated with the 213Bi chelating agent SCN-CHX-A”-diethylenetriaminepentaacetic acid (DTPA) (Macrocyclics, Plano, TX, USA) prior to radiolabelling as previously described16. The α-emitter 213Bi was eluted from an 225Ac/213Bi generator system provided by the Directorate for Nuclear Safety and Security (JRC, EC, Karlsruhe, Germany)12,17. CHX-A”-DTPA–chelated anti-EGFR-MAb (100 µg) was incubated with the 213Bi eluate (37–148 MBq) in 0.4 M ammonium acetate buffer at pH 5.3 for 7 min at room temperature. Unbound 213Bi was separated via size-exclusion chromatography using PD-10 columns (GE Healthcare, Munich, Germany). Purity of 213Bi-anti-EGFR conjugates was controlled as described earlier18. Survival was assessed by microscopical analysis as described previously (see also15).
Treatment of cells with 213 Bi-anti-EGFR-MAb and incubation with [U- 13 C 6 ]glucose
Cells were seeded in 175 cm2 culture flasks (approximately 5 × 106 cells per flask) and allowed to adhere overnight. The next day, cells (approximately 1 × 107 cells per flask) were incubated with lethal activity concentrations of 1.48 MBq/ml of 213Bi-anti-EGFR-MAb in a total volume of 10 ml culture medium for 3 h at 37 °C and 5% CO2. Controls were treated accordingly, however, an equal volume of PBS was added instead of 213Bi-anti-EGFR-MAb solution. Subsequently, culture medium was aspirated and the cells were washed once with 20 ml of PBS. Following addition of 30 ml per flask of [U-13C6]glucose containing culture medium (glucose-free DMEM [Biochrom, Berlin, Germany] with added unlabelled d-glucose [5 mM], [U-13C6]d-glucose [5 mM] [99.9% 13C-content, Sigma-Aldrich, Taufkirchen, Germany], glutamine [0.1 mM] and 10% FCS), the cells were incubated for another 18 h in the incubator (37 °C, 5% CO2). Finally, cells were harvested, washed in PBS three times and the cellular pellets (after removal of the supernatants) were frozen at − 80 °C until further use. Analysis of the cells was done after these 18 h of incubation with [U-13C6]glucose.
Analysis of the 13 C-content of selected amino acids via GC–MS
To analyse treatment associated metabolic alterations in LN18 and EJ28Luc cells upon exposure to 213Bi-anti-EGFR-MAb, mock-treated (controls) and treated cells frozen at − 80 °C were first freeze-dried for 24 h (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode, Germany). For preparation of the amino acids, 1 mL of 6 M HCl was added to 10 mg of freeze-dried cells (approximately 1 × 107) and, after re-suspension, the reaction mixture was incubated for 24 h at 105 °C in a sealed tube. On the following day, the cell hydrolysate was dried at 70 °C in a heat block applying a constant stream of N2 gas. To dissolve the residue, 200 μL of 50% acetic acid were added and the sample was sonicated for 1 min. Then, amino acids were purified using a self-made column composed of a 1 mL pipette tip filled with glass wool and the cation-exchange resin Dowex 50 W-X8 (hydrogen form, 200–400 mesh, Alfa Aesar: L13922, Thermo Fisher Scientific, Waltham, Massachusetts, USA). Before applying the sample, the column was washed once with 1 mL 70% methanol and twice with 1 mL H2O. After addition of the sample, the column was washed twice with 1 mL H2O. The elution of the amino acids was achieved with 1 mL of 4 M NH3 solution. A 200 μL-fraction of the 1 mL eluate was transferred to a glass vial and dried at 70 °C using N2 as described above. The remaining 800 µL of the eluate were kept at − 20 °C for replicate analyses. Amino acids were converted into volatile silyl-derivatives and subjected to GC/MS-analysis, as described earlier19,20. More specifically, 50 μL of water-free acetonitrile (ACN) and 50 μL of N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide (MTBSTFA) containing 1% TBDMS chloride were added and the samples were incubated for 30 min at 70 °C in a sealed GC–MS vial. Finally, samples were transferred to GS–MS microvials and detection of amino acids was done using a GC–MS instrument (GC 2010, GCMS-QP 2010, auto injector AOC-20i, Shimadzu, Munich, Germany).
Data analysis
GC/MS results were evaluated with the associated software GCMSsolution from Shimadzu. Further analysis employed statistical analysis using GraphPad Prism Version 5 (GraphPad Software, Inc., USA). T-Tests were performed followed by Welch’s test. Statistical significance was assumed if p was < 0.05.
Results
Radiochemical yield, specific activity and purity
After a 7-min incubation of the 213Bi eluate with anti-EGFR-MAb, the labeling yield varied between 95 and 97% of 213Bi bound to anti-EGFR-MAb, the respective specific activities were 0.35–1.4 MBq of 213Bi per mg of antibody. After removal of unbound 213Bi via size-exclusion chromatography, a purity of 213Bi-anti-EGFR-MAb of greater than or equal to 99% was achieved. The in vitro stability of 213Bi-anti-EGFR-MAb exceeded four half-lives of 213Bi (3 h), which is in accordance with previous data (see also12).
Effect of 213 Bi-anti-EGFR-MAb treatment on survival of tumor cell lines
The activity concentration of 213Bi-anti-EGFR-MAb applied for incubation of both LN18 glioma and EJ28-luc bladder cancer cells (1.48 MBq/mL) proved to be lethal for approximately 99% of the cells. However, the 3-h treatment with the alpha-emitter immunoconjugate did not eradicate the cells immediately. Cells started to disintegrate not before 72–96 h after incubation with 213Bi-anti-EGFR-MAb and cell death was completed approximately after 120 h. At the time of analysis of the 13C-content of selected amino acids, i.e. 48 h after treatment with 213Bi-anti-EGFR-MAb, cells were still alive, however showed morphological changes compared to mock-treated controls. The most striking difference as observed 48 h after incubation was a massive swelling of the 213Bi-anti-EGFR-MAb treated cells compared to mock-treated controls (data not shown). Likewise, cells that survived 213Bi-anti-EGFR-MAb treatment (approximately 1%) initially underwent a stationary phase of intensive DNA-repair without cell proliferation, lasting approximately 48–72 h.
13 C-Enrichment and isotopologue composition in selected amino acids
As examples for EGFR-overexpressing cancer cells, we have selected the bladder cancer cell line EJ28-Luc and the glioma cell line LN1815. These cell lines were incubated for 3 h with 213Bi-anti-EGFR-Mab and subsequently for 18 h with [U-13C6]glucose. After that, the cells were hydrolysed under acidic conditions. The hydrolysate containing alanine, aspartate, glutamate, glycine, proline and serine was silylated and analysed by GC–MS analysis. By careful evaluation of the mass patterns, the relative abundances of amino acids carrying a given number of 13C-atoms were determined resulting in the isotopologue patterns and overall 13C-contents reported below. Notably, the amino acids under study derive from glycolytic intermediates, such as 3-phosphoglycerate (serine and glycine), pyruvate (alanine), oxaloacetate (aspartate), or α-ketoglutarate (glutamate and proline) following well established pathways in human metabolism (see also Fig. 1). Capitalizing on this fact, impacts of the treatment with 213Bi-anti-EGFR-MAb upon early glucose metabolism (i.e. glycolysis) and downstream carbon fluxes (i.e. citrate cycle) become transparent by this approach.
Cellular metabolism of glucose. The figure shows relevant steps in metabolization of glucose following uptake from the extracellular milieu. Red circles indicate the analyzed amino acids, i.e. alanine (Ala), aspartate (Asp), glutamate (Glu), proline (Pro), serine (Ser), and glycine (Gly), with regard to enrichment of 13C in mock-treated controls and cells incubated with 213Bi-anti-EGFR-MAb. Results of 13C-enrichment in EJ28Luc bladder cancer cells. Red arrows indicate significantly decreased enrichment of 13C in 213Bi-anti-EGFR-MAb treated cells compared to controls. ETC electron transport chain; (adapted from4).
Overall 13 C-contents of amino acids
In EJ28Luc bladder cancer cells, incubation with 213Bi-anti-EGFR-MAb resulted in a significant decrease of overall 13C-incorporation into alanine (3.9% vs 4.5% in controls), into serine (2.9% vs 3.4% in controls), into glutamate (2.7% vs 2.9% in controls), and into aspartate (1.9% vs 2.4% in controls) (Fig. 1, red arrows; Fig. 2A). In contrast, LN18 glioma cells showed no significant decrease of 13C-incorporation into these amino acids (Fig. 2B).
Overall enrichment of 13C in analyzed amino acids of untreated control cells and 213Bi-anti-EGFR-MAb treated cells. Results are shown for EJ28-Luc bladder cancer cells (A) and LN18 glioma cells (B). 213Bi-anti-EGFR-MAb treatment of EJ28-Luc cells resulted in a significant decrease of 13C-enrichment in alanine, aspartate, glutamate, and serine (***p < 0.0001 compared to controls). Moreover, we observed a slight (nonsignificant = n.s.) decrease in proline. Enrichment of 13C varied from 1.6% in proline to 4.5% in alanine (A). In contrast, no significant changes were observed for LN18 cells.
Isotopologue profiles of amino acids from EJ28Luc cells
The most abundant isotopologue of alanine was the [U-13C3]-species containing three (out of three) 13C-atoms (also denoted M + 3, since this isotopologue is characterized by an increase of three mass units due to three 13C-atoms in comparison to the unlabelled species carrying three 12C-atoms (Fig. 3 and Table 1). Glycine, aspartate, glutamate and proline showed the M + 2 isotopologues as the most abundant ones, whereas M + 1 was the dominant one in serine (Fig. 3). The relative amounts of these isotopologues were significantly reduced in 213Bi-anti-EGFR-MAb treated EJ28Luc cells compared to untreated controls in case of alanine (3.6% vs 4.1%), aspartate (1.8% vs 2.12%), glutamate (2.9% vs 3.2%), and serine (2.8% vs 3.2%). However, the differences shown for glycine and proline were not significant (Fig. 3 and Table 1).
Isotopologue profiling with regard to 13C in alanine, aspartate, glutamate, glycine, proline, and serine following incubation of EJ28Luc cells with 213Bi-anti-EGFR-MAb compared to untreated controls. Just as in Fig. 2 showing overall enrichment of 13C, a significantly lower enrichment of 13C in the various C-atoms following 213Bi-anti-EGFR-MAb treatment was only observed for alanine, aspartate, glutamate, and serine but not for glycine and proline. SHMT serine hydroxymethyl transferase, ALT alanine aminotransferase, PC pyruvate carboxylase, PDH pyruvate dehydrogenase.
Isotopologue profiles of amino acids from LN18 cells
In LN18 glioma cells, isotopologue profiling could not reveal significant differences in 13C-enrichments (Fig. 2B) but also not in the isotopologue compositions of the selected amino acids following 213Bi-anti-EGFR-MAb treatment compared to untreated controls (Fig. 4 and Table 1). More specifically, the relative fractions of these isotopologues were not significantly reduced in case of alanine with three 13C labelled atoms (6.3% vs 6.2%), two (out of four) labelled 13C atoms in aspartate (2.8% vs 2.8%) and two (out of five) labelled 13C atoms in glutamate (5.6% vs 5.6%). Accordingly, the differences shown for glycine and proline were not significant. Only the serine isotopologue containing three 13C-atoms showed small, but significant differences (1.9% vs 1.5%; Fig. 4).
Isotopologue profiling with regard to 13C in alanine, aspartate, glutamate, glycine, proline, and serine following incubation of LN18 cells with 213Bi-anti-EGFR-MAb compared to untreated controls. As expected from the results shown in Fig. 2, isotopologue profiling did not reveal significant differences between 213Bi-anti-EGFR-MAb treated and untreated LN18 cells, except for the serine isotopologue containing three 13C-atoms (M + 3). However, the frequency of the different 13C isotopologues was identical for all amino acids in both cell lines analyzed (EJ28Luc and LN18). For example, in proline consisting of five C-atoms, the most frequent isotopologues contained two 13C-atoms and isotopologues with four or five 13C atoms could not be detected in both cell lines. SHMT serine hydroxymethyl transferase, ALT alanine aminotransferase, PC pyruvate carboxylase, PDH pyruvate dehydrogenase.
Discussion
Treatment of cells showing high EGFR expression was chosen so that the 213Bi-anti-EGFR-MAb could bind to the surface of tumor cells, therefore taking advantage of the high linear energy transfer of the alpha emitter 213Bi13,14. We have shown previously that this targeted approach results in effective cell death of both LN18 and EJ28Luc cells15. Other studies revealed that external irradiation can induce enhanced EGFR and MMP-2 secretion in LN18 cells after irradiation with doses ranging from 0.5 to 15 Gy using 6 MV X(-)rays21, thus underlining the principle effect of irradiation in glioma cells. Alpha-particle irradiation with Americium-241, emitting α-particles with an energy of 5.49 meV (LET = 85 keV/μm) at 1.3 Gy resulted in a decrease (albeit not statistically relevant) of relative invasion in an in vitro assay22. In patients with bladder carcinoma overexpressing EGFR, treatment with an 213Bi-anti-EGFR-MAb-conjugate resulted in satisfactory therapeutic efficacy, showing that in three of 12 patients no signs of carcinoma in situ were present at 3, 30 and 44 months after treatment23. Yet, the effects of irradiation using the alpha emitter 213Bi on cell metabolism at a molecular level has not been studied in detail.
Among the hallmarks of cancer is that cancer cells show a high glycolytic rate and lactate production, an anomalous characteristic of cell energy metabolism first described by Otto Warburg24. Cancer cells limit their energy production largely to glycolysis even though oxygen is present by reprogramming their glucose metabolism25. In glioma, a relevant proportion of cell metabolism is supplied through glutamine metabolism, resulting in a so called “glutamine addiction”26.
In our experimental setting, EJ28Luc bladder cancer and LN18 glioma cell lines were cultivated in a medium containing 5 mM of [U-13C6]glucose and 5 mM of unlabelled glucose as well as 0.1 mM glutamine. Given the composition of this cell medium, glycolytic flux might be preferred by the cells due to the low glutamine concentrations. On the other hand, the chosen growth conditions allowed for direct insights into glycolytic flux as a potential early metabolic response upon treatment with 213Bi-anti-EGFR-MAb. Removing glutamine entirely from the medium is challenging as cells deteriorate quickly. In human fibroblasts deficiency of glutamine but not glucose results in apoptosis as shown previously27.
The experimental approach which was used in this study exploits the GC/MS-based 13C-analysis of amino acids for the study of metabolic pathway and fluxes4. Isotopologue patterns and the overall 13C-content in amino acids from the untreated cancer cells under study indeed reflected the 13C-fluxes from the offered 13C-glucose into the respective precursors of these amino acids which also represent central metabolic intermediates involved in glycolysis or the citrate (TCA) cycle (see also Fig. 1).
The labelling data of these untreated cells suggest that glucose uptake and usage via glycolysis and the citrate cycle is more effective in LN18 cells compared to EJ28Luc cells, as gleaned from the higher 13C-content in alanine, aspartate, and glutamate from the 13C-labelled LN18 cells in comparison to the EJ28Luc cells (see Fig. 2). On the other hand, the 13C-content in serine and glycine was lower compared to EJ28Luc cells, possibly indicating a higher use of external (unlabelled) serine by the LN18 cells. This could save NAD+ as serine production from glucose involves two oxidation reactions, which consume NAD+ and produce NADH28. It was shown in A549 cells, in MDA-MB-468 cells, and in MDA-MB-231 cells that these cancer cells need sources of exogenous serine for maximal proliferation28.
With regard to proline, the enrichment was lower in LN18 cells compared to EJ28Luc cells. This could potentially indicate a facilitated metabolism form arginine via ornithine. We hypothesize that this might be necessary for nitrogen mobilization or to provide energy from the TCA cycle. Yet, the direct connection to therapy response is missing.
The 13C-pattern of alanine is of special interest since its direct precursor is pyruvate, the product of glycolysis. Thus, alanine is synthesized from pyruvate by alanine aminotransferase (ALT), hereby inheriting the isotopologue distribution from pyruvate. As pyruvate is mainly synthesized via glycolysis originating from [U-13C6]glucose, the predominant [U-13C3]-isotopologue (M + 3) can be easily understood. Other isotopologues (M + 2 and M + 1) are mainly due to anabolic fluxes from oxaloacetate via gluconeogenesis or from malate via catalytic action of the malic acid enzyme (see Fig. 1). More specifically, phosphoenolpyruvate carboxykinase (PEPCK) provides phosphoenolypyruvate, which can be converted into pyruvate. Consequently, alanine inherits the M + 2 and M + 1 labeling pattern from oxaloacetate. These isotopologues were indeed observed in aspartate (the amination product of oxaloacetate) but also in serine and glycine supporting some metabolic flux via gluconeogenesis although glucose was abundant in the medium. From the observed ratio between M + 3 and M + 2/M + 1 alanine, it can be concluded that glycolysis was the driving force in the malignant cancer cells under study and that fluxes via gluconeogenesis or the malic acid enzyme were negligible (see Table 1).
Quite surprisingly, the impacts of the treatment with 213Bi-anti-EGFR-MAb upon the 13C-labelling patterns described above were highly different for the cell lines under study. Only EJ28Luc cells showed a treatment related decrease of glycolytic flux after incubation with 213Bi-anti-EGFRA-MAb with a statistically significant lower total excess in alanine, aspartate and glutamate enrichment (Fig. 2). Based on the higher glycolytic flux in the LN18 cells and no measurable effects upon the 13C-profiles due to treatment with 213Bi-anti-EGFR-MAb, LN18 cells might in part show a treatment resistance on a metabolic level at least under the experimental conditions used in this study. In line with this hypothesis is that high glucose uptake above the biomass requirements results in an excess production of redox cofactors linked to stress resistance29. In a high-grade human ovarian adenocarcinoma model, it was demonstrated that the cells accumulate micro RNA of the family of miR-200, have low concentrations of p38a, and an associated oxidative stress signature that showed better response to paclitaxel chemotherapy, which is known to increase reactive oxygen species30. However, given that the tumor cells show apoptosis after incubation with 213Bi-anti-EGFR-MAb, it is more likely that disturbance in other processes not related to metabolism lead to lethal cell damage. With the applied methods these processes may not be displayed.
The observation that only the 13C-profiles of EJ28Luc cells showed statistically significant changes due to treatment with 213Bi-anti-EGFR-MAb might indicate that EJ28Luc cells die faster than LN18 cells. A potential reason for this could be that LN18 cells are not as sensitive towards irradiation with the 213Bi-anti-EGFR-MAb immunoconjugate, albeit the fact that these cells eventually deteriorate over time, as shown previously15. This fits to the previous observation that [18F]FDG uptake did not decrease statistically significant in LN18 cells, however in EJ28Luc cells after irradiation with a 213Bi-anti-EGFR-MAb15. Interestingly, [18F]FDG uptake was slightly higher in LN18 cells compared to EJ28 Luc cells prior to treatment (ca. 38% vs. ca. 30%)15. The latter finding might be a result of enhanced glucose (GLUT) transporter capacity. It was shown in human and murine glioblastoma cells that by inhibition of GLUT/SLC2A with indinavir, ritonavir and by inhibition of the Na/glucose antiporter type 2 (SGLT2/SLC5A2) superfamily with phlorizin, glucose consumption and cell proliferation were decreased31. In C6 glioma cells, glutamine metabolism appeared complementary to that of glucose with respect to energy production as carbon donor to replenish the tricarboxylic acid cycles32. In order to sustain TCA cycle function, oxaloacetate needs to be refilled by anapleurosis33, for example by carboxylation of pyruvate or phosphoenol pyruvate. This allows cells to use the TCA cycle as a supply of biosynthetic precursors33. In glioma, metabolism from pyruvate to oxaloacetate seems to be decreased as indicated by the ratio of pyruvate carboxylase/PDH activity compared to normal glia and neuronal tissue34. In cancer cells, a more relevant anaplerotic pathway appears to be driven by the usage of glutamine, which contributes to proliferation and biosynthesis35,36. Generally, in proliferating glioma cells glutamine is mainly utilized for anapleurosis as carbon donor to replenish the tricarboxylic acid cycle as demonstrated by 13C nuclear magnetic resonance spectroscopy in C6 glioma cells32 and in human SF188 glioblastoma cells37. In cells depleted of glutamine, pools of fumarate, malate and 5-oxo-proline were significantly decreased and these cells went into apoptosis27.
Interestingly, EJ28Luc and LN18 cells showed highly similar relative abundance of the different 13C isotopologues. Given that both cell lines represent totally different cancer types, this is noteworthy, allowing potentially for a similar therapeutic approach.
Using the approach with [U-13C6]glucose, deeper insights into cellular metabolism might become possible beyond the first step of glycolysis as accessible with [18F]FDG-PET imaging, since [18F]FDG gets trapped after phosphorylation through hexokinase. Moreover, approximately 30% of cancers cannot be detected by [18F]FDG-PET, either because these tumors do not show a glucose uptake that is above the limit of detection or the tumor entity relies on other metabolic pathways to fuel energy demands38.
Further steps of the glycolytic pathway are not directly accessible using [18F]FDG-PET imaging. A possible approach to gain further insights is the use of 13C-enriched glucose or intermediates of glycolysis such as 13C-labeled pyruvate. Labelling with 13C takes advantage of the fact that naturally 13C is low in abundance (ca. 1%)39 and therefore can be used to investigate metabolism using magnetic resonance spectroscopy (NMR). At thermal equilibrium, these measurements require relatively long time to obtain a reasonable signal to noise ratio (SNR) due to the inherently low sensitivity of NMR. Dynamic nuclear hyperpolarization showed to improve SNR by > 10,000-fold and allowed for in vitro and in vivo characterization of metabolic processes40. Among the most frequently used substances is 13C-pyruvate, which allows to describe metabolic alterations of cancer cells through measurement of pyruvate to lactate metabolism in vitro and in vivo15,41,42,43. In principle, this is also possible with hyperpolarized 13C-enriched glucose as shown before44. However, the short T1 time of 8–10 s of pre-deuterated hyperpolarized 13C-enriched glucose makes it very challenging to follow several steps of glycolysis in vitro and in vivo using NMR45. Within this short time interval, the substance has to be dissolved and has to be given to the cells or patients. Another approach was recently proposed using chemical shift imaging (CSI) experiments using magnetic resonance imaging (MRI)46. By improving the post-processing procedure by higher dimensional analog of singular value decomposition, tensor decomposition, an approximately 30-fold increase of SNR could be achieved in CSI experiments46. We plan to establish such a protocol in future in order to be able to take advantage of this approach. To further explore which metabolic intermediates might be potentially interesting for such a “metabolic” approach, we also aimed to understand the metabolic roads of glycolytic intermediates using the 13C-approach monitored by GC–MS. This approach starting from [U-13C6]glucose allows for a precise quantification of cell metabolism without additional hurdles of fast decay of signals as for instance with hyperpolarized 13C-glucose. Given that EJ28Luc cells show changes of glycolytic intermediates leading to amino acids under study, this may enable future “focused” studies using also hyperpolarized 13C glucose or non-hyperpolarized magnetic resonance spectroscopy using CSI.
Beyond these aspects, the pilot experiments in this study show the effects of the alpha-emitter 213Bi upon cell metabolism in considerable detail. This is of relevance, as it was shown previously that metformin and temozolomide enhanced the effectiveness of photon irradiation in LN18 glioblastoma cells47. This coincided with G2/M cell cycle arrest and changes in pAMPK levels possibly triggering cell metabolism47. As treatment of glioblastoma remains to be challenging, identification of new “metabolic” targets might provide new treatment options. Combination of external or internal radiation with targeted therapies might enhance treatment success. In the invasive bladder carcinoma cell line EJ28, it was demonstrated that, in varying conditions of confluence and hypoxia (0.1% O2), hypoxia significantly increased VEGF protein expression, which correlated with expression of the transcription factors hypoxia inducible factor (HIF) HIF1-alpha and HIF-2-alpha having again impacts on the core metabolism of these cells48.
By a better understanding of treatment related effects on a metabolic level, new therapeutic targets could be identified. However, the 13C-approach described here has limitations. For example, a direct monitoring using intact cells is still not possible. Additionally, the method requires a significant amount of time in order to prepare and analyse treatment-related effects even under in vitro conditions. This might limit its broad application in a pre-clinical and clinical setting. On the other hand, also [18F]FDG-PET imaging in glioma and also in bladder cancer cannot be easily applied: human brain metabolism largely relies on glucose metabolism and therefore shows high background uptake of [18F]FDG; besides [18F]FDG is excreted via the urinary tract and therefore accumulates in the bladder, resulting in the difficulty of identifying the tumour. In the case of glioma, the use of 4-18F-(2S, 4R)-fluoroglutamine was proposed for PET imaging of glioma, offering the advantage of low background uptake through imaging of glutamine uptake49.
To our knowledge, this is the first study that evaluated the effects of alpha-irradiation using 213Bi-anti-EGFR-MAb on cellular metabolism. Targeted treatment with alpha emitters is recently gaining more interest, e.g. for prostate cancer using 225Ac-PSMA-617. Nevertheless, studies show that although initial good treatment effects can be achieved, some patients show progressive disease eventually50,51,52. Thus, our results underscore the importance of a better understanding of cellular metabolism with the aim of potentially identifying new targets for combination treatments to enhance treatment success.
Conclusions
Treatment of EJ28Luc bladder cancer cells, but not of the LN18 glioma cells with 213Bi-anti-EGFR-MAb resulted in decreased incorporation of 13C-labelled glucose into amino acids derived from glycolytic and TCA cycle intermediates, indicative of an early treatment response. Our pilot experiments show that the 13C-labelling approach can be a valid tool to assess the responsivity of cancer cells upon targeted alpha radionuclide treatment at high metabolic resolution.
Data availability
Data generated or analyzed during this study are included in this published article.
References
Warburg, O. H. The classic: The chemical constitution of respiration ferment. Clin. Orthop. Relat. Res. 468, 2833–2839. https://doi.org/10.1007/s11999-010-1534-y (2010).
Smith, T. A. The rate-limiting step for tumor [18F]fluoro-2-deoxy-d-glucose (FDG) incorporation. Nucl. Med. Biol. 28, 1–4. https://doi.org/10.1016/s0969-8051(00)00177-3 (2001).
Eisenreich, W., Huber, C., Kutzner, E., Knispel, N. & Schramek, N. Isopologue Profiling-Toward a Better Understanding of Metabolic Pathways in The Handbook of Plant Metabolomics Ch. 2, 25–56 (2013).
Gkiouli, M., Biechl, P., Eisenreich, W. & Otto, A. M. Diverse roads taken by (13)C-glucose-derived metabolites in breast cancer cells exposed to limiting glucose and glutamine conditions. Cells https://doi.org/10.3390/cells8101113 (2019).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528. https://doi.org/10.1016/j.cmet.2016.01.007 (2016).
Fan, T. W. et al. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM). Mol. Cancer 8, 41. https://doi.org/10.1186/1476-4598-8-41 (2009).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694. https://doi.org/10.1016/j.cell.2015.12.034 (2016).
Phannasil, P. et al. Pyruvate carboxylase is up-regulated in breast cancer and essential to support growth and invasion of MDA-MB-231 cells. PLoS ONE 10, e0129848. https://doi.org/10.1371/journal.pone.0129848 (2015).
Zhou, X. et al. PROTOCADHERIN 7 acts through SET and PP2A to potentiate MAPK signaling by EGFR and KRAS during lung tumorigenesis. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-16-1267-T (2016).
Trivedi, S. et al. Anti-EGFR targeted monoclonal antibody isotype influences antitumor cellular immunity in head and neck cancer patients. Clin. Cancer Res. 22, 5229–5237. https://doi.org/10.1158/1078-0432.CCR-15-2971 (2016).
Rappl, A., Piontek, G. & Schlegel, J. EGFR-dependent migration of glial cells is mediated by reorganisation of N-cadherin. J. Cell Sci. 121, 4089–4097. https://doi.org/10.1242/jcs.027995 (2008).
Pfost, B. et al. Intravesical alpha-radioimmunotherapy with 213Bi-anti-EGFR-mAb defeats human bladder carcinoma in xenografted nude mice. J. Nucl. Med. 50, 1700–1708. https://doi.org/10.2967/jnumed.109.065961 (2009).
Seidl, C. Radioimmunotherapy with alpha-particle-emitting radionuclides. Immunotherapy 6, 431–458. https://doi.org/10.2217/imt.14.16 (2014).
Morgenstern, A. et al. An overview of targeted alpha therapy with (225)Actinium and (213)Bismuth. Curr. Radiopharm. 11, 200–208. https://doi.org/10.2174/1874471011666180502104524 (2018).
Feuerecker, B. et al. Assessment of (213)Bi-anti-EGFR MAb treatment efficacy in malignant cancer cells with [1-(13)C]pyruvate and [(18)F]FDG. Sci. Rep. 9, 8294. https://doi.org/10.1038/s41598-019-44484-w (2019).
Mirzadeh, S., Brechbiel, M. W., Atcher, R. W. & Gansow, O. A. Radiometal labeling of immunoproteins: covalent linkage of 2-(4-isothiocyanatobenzyl)diethylenetriaminepentaacetic acid ligands to immunoglobulin. Bioconjug. Chem. 1, 59–65 (1990).
Apostolidis, C., Molinet, R., Rasmussen, G. & Morgenstern, A. Production of Ac-225 from Th-229 for targeted alpha therapy. Anal. Chem. 77, 6288–6291. https://doi.org/10.1021/ac0580114 (2005).
Seidl, C. et al. Cell death triggered by alpha-emitting 213Bi-immunoconjugates in HSC45-M2 gastric cancer cells is different from apoptotic cell death. Eur. J. Nucl. Med. Mol. Imaging 32, 274–285. https://doi.org/10.1007/s00259-004-1653-3 (2005).
Eylert, E. et al. Isotopologue profiling of Legionella pneumophila: Role of serine and glucose as carbon substrates. J. Biol. Chem. 285, 22232–22243. https://doi.org/10.1074/jbc.M110.128678 (2010).
Eylert, E. et al. Carbon metabolism of Listeria monocytogenes growing inside macrophages. Mol. Microbiol. 69, 1008–1017. https://doi.org/10.1111/j.1365-2958.2008.06337.x (2008).
Martinou, M. et al. Ionizing radiation affects epidermal growth factor receptor signalling and metalloproteinase secretion in glioma cells. Cancer Genom. Proteom. 8, 33–38 (2011).
Wank, M. et al. Evaluation of radiation-related invasion in primary patient-derived glioma cells and validation with established cell lines: Impact of different radiation qualities with differing LET. J. Neurooncol. 139, 583–590. https://doi.org/10.1007/s11060-018-2923-4 (2018).
Autenrieth, M. E. et al. Treatment of carcinoma in situ of the urinary bladder with an alpha-emitter immunoconjugate targeting the epidermal growth factor receptor: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 45, 1364–1371. https://doi.org/10.1007/s00259-018-4003-6 (2018).
Warburg, O. H., Dickens, F. Kaiser-Wilhelm-Institut f¸r, B. The metabolism of tumours; investigations from the Kaiser Wilhelm institute for biology, Berlin-Dahlem. (Constable & Co. Ltd., 1930).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144, 646–674. https://doi.org/10.1016/j.cell.2011.02.013 (2011).
Marquez, J. et al. Glutamine addiction in gliomas. Neurochem. Res. 42, 1735–1746. https://doi.org/10.1007/s11064-017-2212-1 (2017).
Yuneva, M., Zamboni, N., Oefner, P., Sachidanandam, R. & Lazebnik, Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 178, 93–105. https://doi.org/10.1083/jcb.200703099 (2007).
Diehl, F. F., Lewis, C. A., Fiske, B. P. & Vander Heiden, M. G. Cellular redox state constrains serine synthesis and nucleotide production to impact cell proliferation. Nat. Metab. 1, 861–867. https://doi.org/10.1038/s42255-019-0108-x (2019).
Zielinski, D. C. et al. Systems biology analysis of drivers underlying hallmarks of cancer cell metabolism. Sci. Rep. 7, 41241. https://doi.org/10.1038/srep41241 (2017).
Mateescu, B. et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat. Med. 17, 1627–1635. https://doi.org/10.1038/nm.2512 (2011).
Azzalin, A. et al. Inhibitors of GLUT/SLC2A enhance the action of BCNU and temozolomide against high-grade gliomas. Neoplasia 19, 364–373. https://doi.org/10.1016/j.neo.2017.02.009 (2017).
Portais, J. C., Voisin, P., Merle, M. & Canioni, P. Glucose and glutamine metabolism in C6 glioma cells studied by carbon 13 NMR. Biochimie 78, 155–164. https://doi.org/10.1016/0300-9084(96)89500-9 (1996).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20. https://doi.org/10.1016/j.cmet.2007.10.002 (2008).
Brand, A., Engelmann, J. & Leibfritz, D. A 13C NMR study on fluxes into the TCA cycle of neuronal and glial tumor cell lines and primary cells. Biochimie 74, 941–948. https://doi.org/10.1016/0300-9084(92)90078-s (1992).
Kovacevic, Z. & McGivan, J. D. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol. Rev. 63, 547–605. https://doi.org/10.1152/physrev.1983.63.2.547 (1983).
Eagle, H., Oyama, V. I., Levy, M., Horton, C. L. & Fleischman, R. The growth response of mammalian cells in tissue culture to l-glutamine and l-glutamic acid. J. Biol. Chem. 218, 607–616 (1956).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 104, 19345–19350. https://doi.org/10.1073/pnas.0709747104 (2007).
Jones, R. G. & Thompson, C. B. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 23, 537–548. https://doi.org/10.1101/gad.1756509 (2009).
Eakin, R. T., Morgan, L. O., Gregg, C. T. & Matwiyoff, N. A. Carbon-13 nuclear magnetic resonance spectroscopy of living cells and their metabolism of a specifically labeled 13C substrate. FEBS Lett. 28, 259–264. https://doi.org/10.1016/0014-5793(72)80726-9 (1972).
Serrao, E. M. & Brindle, K. M. Dynamic nuclear polarisation: The future of imaging in oncology?. Porto Biomed. J. 2, 71–75. https://doi.org/10.1016/j.pbj.2017.01.002 (2017).
Feuerecker, B. et al. Hyperpolarized (13)C diffusion MRS of co-polarized pyruvate and fumarate to measure lactate export and necrosis. J. Cancer 8, 3078–3085. https://doi.org/10.7150/jca.20250 (2017).
Bliemsrieder, E. et al. Hyperpolarized (13)C pyruvate magnetic resonance spectroscopy for in vivo metabolic phenoty** of rat HCC. Sci. Rep. 11, 1191. https://doi.org/10.1038/s41598-020-80952-4 (2021).
Hundshammer, C. et al. Simultaneous characterization of tumor cellularity and the Warburg effect with PET, MRI and hyperpolarized (13)C-MRSI. Theranostics 8, 4765–4780. https://doi.org/10.7150/thno.25162 (2018).
Mishkovsky, M. et al. Measuring glucose cerebral metabolism in the healthy mouse using hyperpolarized (13)C magnetic resonance. Sci. Rep. 7, 11719. https://doi.org/10.1038/s41598-017-12086-z (2017).
Singh, J. et al. Probing carbohydrate metabolism using hyperpolarized (13) C-labeled molecules. NMR Biomed. 32, e4018. https://doi.org/10.1002/nbm.4018 (2019).
Brender, J. R. et al. Dynamic imaging of glucose and lactate metabolism by (13)C-MRS without hyperpolarization. Sci. Rep. 9, 3410. https://doi.org/10.1038/s41598-019-38981-1 (2019).
Adeberg, S. et al. Metformin enhanced in vitro radiosensitivity associates with G2/M cell cycle arrest and elevated adenosine-5’-monophosphate-activated protein kinase levels in glioblastoma. Radiol. Oncol. 51, 431–437. https://doi.org/10.1515/raon-2017-0042 (2017).
Jones, A. et al. Relation of vascular endothelial growth factor production to expression and regulation of hypoxia-inducible factor-1 alpha and hypoxia-inducible factor-2 alpha in human bladder tumors and cell lines. Clin. Cancer Res. 7, 1263–1272 (2001).
Venneti, S. et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci. Transl. Med. 7, 274ra217. https://doi.org/10.1126/scitranslmed.aaa1009 (2015).
Sathekge, M. et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving 225 Ac-PSMA-617 radioligand therapy. J. Nucl. Med. https://doi.org/10.2967/jnumed.119.229229 (2019).
Kratochwil, C. et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: Swimmer-plot analysis suggests efficacy regarding duration of tumor control. J. Nucl. Med. 59, 795–802. https://doi.org/10.2967/jnumed.117.203539 (2018).
Feuerecker, B. et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur. Urol. https://doi.org/10.1016/j.eururo.2020.11.013 (2020).
Acknowledgements
The 225Ac/213Bi generator system was kindly provided by the Joint Research Centre, European Commission, Directorate for Nuclear Safety and Security, Karlsruhe, Germany. The authors would like to express their gratitude to the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich SFB 824, Bonn, Germany) for support. P.B. and W.E. also thank the Wilhelm Sander-Stiftung (Munich, Germany) for support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All listed authors have actively contributed to this work. B.F., P.B. and C.S. performed the experiments. B.F., W.E. and C.S. designed the study and drafted the manuscript. M.S. participated in the study design and helped to draft the manuscript. P.B. performed analysis of GC/MS. F.B. prepared the 225Ac/213B generator system, A.M. controlled and approved the application and revised the manuscript. B.F. performed analysis of the data and C.S. prepared 213Bi-anti-EGFR-MAb experiments, wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feuerecker, B., Biechl, P., Seidl, C. et al. Diverse metabolic response of cancer cells treated with a 213Bi-anti-EGFR-immunoconjugate. Sci Rep 11, 6227 (2021). https://doi.org/10.1038/s41598-021-84421-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84421-4
- Springer Nature Limited
This article is cited by
-
Targeted Alpha Therapy for Glioblastoma: Review on In Vitro, In Vivo and Clinical Trials
Targeted Oncology (2024)