Abstract
Mild traumatic brain injury is an all-too-common outcome from modern warfare and sport, and lacks a reproducible model for assessment of potential treatments and protection against it. Here we consider the use of surface acoustic wave (SAW) irradiation of C. elegans worms—without cavitation—as a potential, ethically reasonable animal-on-a-chip model for inducing traumatic brain injury in an animal, producing significant effects on memory and learning that could prove useful in a model that progress from youth to old age in but a few weeks. We show a significant effect by SAW on the ability of worms to learn post-exposure through associative learning chemotaxis. At higher SAW intensity, we find immediate, thorough, but temporary paralysis of the worms. We further explore the importance of homogeneous exposure of the worms to the SAW-driven ultrasound, an aspect poorly controlled in past efforts, if at all, and demonstrate the absence of cavitation through a change in fluids from a standard media for the worms to the exceedingly viscous polyvinyl alcohol. Likewise, we demonstrate that acoustic streaming, when present, is not directly responsible for paralysis nor learning disabilities induced in the worm, but is beneficial at low amplitudes to ensuring homogeneous ultrasound exposure.
Similar content being viewed by others
Introduction
The increased incidence of reported blast-related head injuries has made traumatic brain injury (TBI) the signature injury of modern warfare1. TBI does not discriminate by age, socioeconomic group, nor gender, and as a significant health and economic burden2,3, it affects over 1.7 million people in the United States each year4. TBI can produce a wide array of acute symptoms in moderate-to-severe exposure, but blast-induced mild traumatic brain injury (bi-mTBI) is characterized by the distinct absence of acute clinical abnormalities. Although exact figures are unknown, approximately one in five wounded soldiers suffers from TBI5, and an estimated 52% of those injuries are bi-mTBI that occur over both short- and long-term scales6,7,8. In both civilian and military environments, exposure to a blast may cause instant death, injuries with immediate sign of symptoms, or concealed injuries manifesting over years to decades after the initial blast exposure9. Bi-mTBI may underlie a risk of later develo** neurodegenerative disorders, such as Alzheimer’s disease10,11, chronic traumatic encephalopathy12,13, depression14,15,16,17,18,19,20, and neural, axonal, and glial tissue injury21,22.
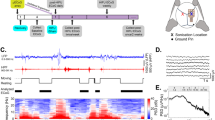
The injuries suffered as a result of the blast exposure may serve to define the mechanisms of brain injury. Bi-mTBI is divided into four subcategories: the primary injury is the result of the direct effect of a blast wave, the secondary injury is due to the victim’s physical contact with blast-scattered fragments, the tertiary injury results from acceleration of the head due to wind from the blast and being struck by objects, and chemical and thermal blast effects deliver the quaternary injury23,64. Notably, the approximately linear reduction in the worms’ movement speed with increasing SAW power (Fig. 1(e)) is apparent all the way to 1000 mW in the thin film configuration, while the trend is weaker in the chamber at any power, and fails above 500 mW. Most importantly, otherwise motile and normal naïve worms as shown in Fig. 1(e) appeared essentially the same after 10 s of exposure to ~500 mW SAW (see Fig. 1(f)), with reduced motility as the only evidence of SAW exposure.
By contrast, the worms were completely paralyzed and straight from only 5 s of exposure to 1000 mW of SAW (see Fig. 1(g)) in the sessile droplet configuration (Fig. 1(c)). No paralysis of this form was ever observed from 10 s of exposure in the chamber (Fig. 1(a,b)) at any input power.
We seek to avoid paralysis while obtaining a consistent reduction in worm motility, believed to represent the sub-critical, principally behavioral injury in worms similar to bi-mTBI observed in humans and mammals: loss of function (mobility) without other obvious impairment. Consequently, 500 mW was chosen as the optimum SAW power while using the exposure chamber. This choice struck a balance between maximizing direct irradiation and minimizing acoustic streaming to achieve the maximum reduction in the worm’s speed of movement, a key loss of function, while simultaneously aiming to minimize the number of paralyzed worms. While the thin fluid film results are interesting in their own way, the problems of evaporation and difficulty in fluid handling prevented this configuration from being considered for the remainder of this study. The recovery of the worms’ mobility will be explored in the following section.
Recovery of C. elegans’ mobility after SAW exposure
As illustrated in Fig. 2, the worms’ mobility fully recovered within about 1 hour after 10 s of 20-MHz, 500 mW SAW exposure and deposition upon an agar plate, showing an average movement speed statistically indistinguishable (ns) to unexposed (control) worms. The average speed reported immediately after deposition upon the agar plate at 0 hours is much lower than after 1 or 2 hours, but this is in significant part due to the initial crowding of the worms. The worms were confluent in the M9 buffer before placement upon the agar, and so were constrained in their initial motion by crowding after placement; the control also exhibited a reduction in motility as a consequence. However, despite this effect, a significant reduction in worm motility was observed immediately after exposure (0 hours) that is directly attributable to SAW exposure.
C. elegans motility recovers after 10 s of 20-MHz, 500 mW SAW exposure to become statistically indistinguishable with unexposed (control) worms in about one hour. Crowding (see text) was partially responsible for the initial reduction in motility for the control and 500-mW SAW-exposed worms, but a significant reduction in motility was observed due to SAW exposure alone. Mean (S.E.) values were obtained from N = 4 independent experiments, with n = 150–200 worms tested in each experiment (p < 0.05). Significance was based upon a two-way ANOVA over the n individual worm data and all N independent experiments for each reported time with \(\alpha =0.05\), where ns implies p > 0.05, *p < 0.05, **p < 0.01, and ***p < 0.0005. Normality of the data was assessed using the Shapiro-Wilk test with *p > α indicating the experimental data was normally distributed.
It is important to ensure the exposed worms recover their mobility before being used for associative learning and memory assays that require worms to move along a certain distance and reach designated spots, so that the worms’ behavior is assessed in a manner analogous to the clinical assessment of bi-mTBI in humans, such as short-term memory loss and learning65. If the worms are mobile but still cannot move to designated spots, it is not because of the loss of mobility but instead is due to damage to their memory and ability to learn, the same as those observed in humans and mammals exposed to shock waves. Just as it would be unreasonable to judge a human’s brain impairment in a walking course with a broken leg, it is unreasonable to use reduced mobility worms for assays that conflate mobility with learning and memory.
Effect of SAW on C. elegans’ learning and short-term memory: a proof-of-concept
As illustrated in Fig. 3, C. elegans worms unexposed to SAW showed a short-term memory profile consistent with past observations65. That is, after conditioning, wild-type worms’ chemotaxis to butanone increased significantly (****p < 0.0002) immediately after conditioning in comparison to their naïve state. This response to conditioning—typically associated with their memory of the training—was gradually lost over the next two hours, with an insignificant change (ns, p > 0.05) from both zero to one hour post-conditioning and one to two hours post-conditioning.
Chemotaxis assay (a–f) procedure and (g) results. The origin (at bottom), butanone (at left), and EtOH (at right) spots were (a) marked underneath a transparent plate. (b) First, 1 μL NaN3 was added to each of the butanone and EtOH spots. (c) Then 1 μL each of 10% butanone and 95% EtOH were respectively added to the spots on the left and right sides of the plate. (d) Immediately after being trained via the learning assay, 200 to 400 worms suspended in M9 buffer were then added to the plate over the “origin”. Upon reaching the (e) butanone and (f) EtOH spots, the worms were paralyzed and counted. In this way, the effect of 10 s of 500 mW 20-MHz SAW on associative learning and short-term associative memory of C. elegans worms may be (g) compared to worms unexposed to SAW. Unexposed worms showed significant learning ability (****p < 0.0002), and their short-term memory lasts for ~2 hours with an insignificant decline in their learning ability over that time (ns, p > 0.05 for 0–1 hr and 1–2 hrs). SAW-exposed worms exhibited a weakly significant change in the massed chemotaxis index (*p < 0.05) post-conditioning. The SAW-exposed worms showed a continued increase in learning ability to 1 hour after conditioning (*p < 0.05), followed by a decrease in learning ability from 1 to 2 hours after conditioning (*p < 0.05). The massed chemotaxis index significantly increased with respect to the unexposed (naïve) worms one hour after training (**p < 0.007). The naïve–0 hr, naïve–1 hr, and naïve–2 hr runs were each repeated four times (N = 4) for both the control and SAW-exposed conditions, with n = 200–400 worms for each and every repeat. Significance was based upon the entire data set with a two-way ANOVA with \(\alpha =0.05\), where *p < 0.05, **p < 0.007, ***p < 0.0005 and ****p < 0.0002. Normality of the data was assessed using the Shapiro-Wilk test with *p > α indicating the experimental data was normally distributed.
However, worms exposed to SAW showed different learning and short-term memory patterns. After conditioning, they exhibited only weakly significant (*p < 0.05) learning, and this learning actually increased in the hour after conditioning (*p < 0.05) and decreased from one to two hours post-conditioning (*p < 0.05). Further, the massed chemotaxis index correlated with their short-term memory differed between the exposed and unexposed worms: the SAW-exposed worms expressed the highest massed chemotaxis index fully one hour after training, in an apparently delayed but significant (***p < 0.007) learning response from conditioning.
Absence of cavitation in SAW-induced reduction of C. elegans’ mobility
Cavitation—the formation and collapse of vapor bubbles from induced high positive pressure followed by negative pressure— and its resulting mechanical impact on C. elegans has been used to damage the neuronal structure and decrease C. elegans’ mobility52,53. Surprisingly, with SAW no such effect was seen. Cavitation, if present, was found to have no significant effect on the mobility of worms exposed to SAW and its associated high acceleration (10–100 Mm/s2) sound waves generated in the fluid surrounding the worms. While our calculations suggest the threshold frequency for cavitation induced by any nucleation site of 10 nm or larger in water is 4.6 MHz, based on the classic work of Neppiras and Noltingk63, the importance of the matter requires confirmation that cavitation is absent, or, if present, is not contributing to the observations.
The propensity to induce cavitation (if any) by SAW was assessed by simply replacing the M9 buffer with polyvinyl alcohol (PVA), which has much greater viscosity. Consequently, this change greatly reduces the induction, appearance, and intensity of cavitation66,67, while maintaining nearly the same acoustic impedance as water68. The speed of the worms remained similar despite the change in fluid, whether or not they were exposed to 500 mW SAW in the chamber (Fig. 4(a)).
To determine if cavitation is a factor in the observed reduction in C. elegans’ motility, we exposed the worms to 10 s of 500 mW 20-MHz SAW in \(10\,\mu \ell \) droplets of either polyvinyl alcohol or M9 solutions in the chamber configuration (see Fig. 1(a,b)). Polyvinyl alcohol, as prepared, is far more viscous than the M9 buffer solution with a consequent reduction in any ability to induce cavitation. The (a) effects of the SAW on the worms’ average speed in the two solutions were statistically identical (ns, p > 0.5), whether exposed to SAW or not. For (a), significance was based upon a two-way ANOVA upon data from \(N=12\) independent experiments for the control and for the SAW-exposed worms, with n = 150–200 worms tested in each experiment, defining **p < 0.002. By increasing the SAW power to 1500 mW and switching to the (b) free fluid film configuration (see Fig. 1(c)), many of the worms were found to be paralyzed after only 5 s exposure, whether in M9, PVA 5% wt, or PVA 15% wt solutions. The (c) ratio of paralyzed to total number of worms in each experiment shows that switching from the less viscous M9 to the more viscous PVA produces a significantly (**p < 0.01) greater amount of paralysis, with insignificant (ns, p > 0.5) differences in paralysis between 5% wt and 15% wt solutions of PVA. Significance was based upon a two-way ANOVA upon \(N=6\) independent experiments for each of the three liquid media choices, with n = 100–150 worms tested in each experiment, where ns implies p > 0.05, *p < 0.05, and **p < 0.01. In both (a,c) experiments, the normality of the data was assessed using the Shapiro-Wilk test with *p > α = 0.05 indicating the data were normally distributed.
By switching from the chamber configuration to the thin-film fluid configuration, and increasing the SAW power from 500 to 1000 mW, many of the worms were paralyzed. Figure 4(b,c) shows the worms’ paralysis regardless of whether M9, PVA 5% wt, or PVA 15% wt solutions were used. Interestingly, the worms’ paralysis increased with increasing viscosity, significantly so (**p < 0.01) in the switch from M9 to PVA. By contrast, switching between 5% wt and 15% wt solutions of PVA produced insignificant (ns, p > 0.5) differences in paralysis. If cavitation were a factor in the paralysis, the greater propensity of cavitation in the M9 would be expected to produce a greater number of paralyzed worms. The opposite occurs.
One aspect that must be checked is the relative acoustic impedance of these fluids. Potentially, the acoustic impedance \(Z={\rho }_{f}{c}_{f}\), a product of the density \({\rho }_{f}\) and the speed of sound cf, could be so different between them that the effective transmission of acoustic energy into the fluid from the SAW could likewise be different, producing unexpected results. For example, perhaps the observed results are due to the PVA having a much lower acoustic impedance, with far less energy passing into the fluid from the SAW in the substrate.
To determine the acoustic impedance, we require the density and speed of sound. The density of each of the fluids is straightforward to measure, while the speed of sound may be determined from the Rayleigh angle \({\theta }_{{\rm{R}}}={\sin }^{-1}\,{c}_{f}/{c}_{{\rm{SAW}}}\) where cf and cSAW are the phase velocities of the sound in the fluid and of the SAW in the substrate, respectively: \({c}_{f}={c}_{{\rm{SAW}}}\,\sin \,{\theta }_{{\rm{R}}}\). The Rayleigh angle can be determined by measuring the angle of acoustic streaming produced within the fluid placed upon the SAW-generating substrate, visualized by dye tracing the flow from the acoustic source. In our measurements, deionized water (DIW), M9 buffer and PVA 5% weight produce Rayleigh angles of 22.0° ± 0.5°, 24.0° ± 0.5°, and 27.0° ± 0.5°. Since the SAW velocity is 3990 m/s in the LN substrate, this produces a sound speed in the fluid of cf = 1490, 1620, and 1810 m/s, respectively, all ±30 m/s. The PVA solution therefore has a higher acoustic impedance than DIW and M9 as a result of its greater density and sound speed. This actually improves the transmission of sound into the fluid from the SAW in the substrate, so the change in the acoustic impedance is not responsible for the observations.
Discussion
If acoustic streaming could be considered to be a contributing factor to the worms’ paralysis, it is insignificant (Fig. 4). Acoustic streaming is much weaker as the viscosity is increased from M9 to the PVA solutions, particularly the 15% wt solution, yet the paralysis effects are stronger with increasing viscosity. Furthermore, changes in the acoustic impedance were shown to not contribute to the observed effects. Finally, we find that cavitation is not responsible for the observed effects.
Therefore, it can be concluded that the sole remaining contributor from the SAW on the worms, large acceleration from the acoustic radiation propagating through the fluid, is the main injurious component. This effect appears to be responsible for the observed paralysis at high SAW intensity and the observed learning delays at lower SAW intensity induced in our worm-on-a-chip device. However, it is important to remember that acoustic streaming can aid in improving the homogeneity of the C. elegans’ exposure to the ultrasound by transporting them across the testing chamber, and can therefore be beneficial in improving the consistency of the results one may obtain.
Exposure to SAW-driven high frequency ultrasound affects the worms’ ability to move, with paralysis and morphological changes consistent with results shown in the literature54. Though past studies have indicated ultrasound appears to have some effect on C. elegans behavior and morphology52,53,54, in those studies where more than a few worms have been utilized, the results have lacked statistical power likely due to the heterogeneity of the ultrasound that the worms have been exposed to.
We furthermore show that exposure to SAW-induced ultrasound significantly affects associative learning and short-term memory, and that neither cavitation nor acoustic streaming is responsible for the observed effects, producing a potentially useful model for bi-mTBI in demonstrating a delayed learning ability after conditioning due to SAW exposure.
Regardless, the mechanism inducing these effects remains unknown. Further work is needed—and several studies are underway69—to determine the mechanisms underpinning ultrasonic neuromodulation before it may be considered a useful tool in neuroscience research. The current study indicates that SAW may prove to be useful in conjunction with C. elegans in studies that require the induction of significant effects in learning and memory.
Methods
Exposure apparatus
As depicted in Fig. 1, a single port, 25-finger pair interdigital transducer (IDT) 20 MHz SAW device with a 5 mm aperture was used in this work. The widths of the fingers and gaps between them were set at λ/4 = 50 μm to define the SAW wavelength at λ = 200 μm. Double-side polished 500 μm thick, 127.68° y-rotated cut, x-axis propagating single-crystal lithium niobate (LN) was used for the substrate. Sputter deposition of 10 nm Cr/1 μm Al was followed by ultraviolet photolithography with AZ 1518 photoresist (Microchem, Westborough, MA) and AZ 400 K developer (Microchem, Westborough, MA) and wet etching to fabricate the IDTs. The Rayleigh surface acoustic wave (Fig. 1(b,c)) was generated from a sinusoidal electric field generated by a signal generator (N9310A, Agilent Technologies, Santa Clara, CA) and applied via an amplifier (10W1000C, Amplifier Research, Bothell, WA). The input electrical signals were measured at the device using an oscilloscope (Wavejet 332/334, LeCroy, Chestnut Ridge, NY, USA), and the resulting Rayleigh wave was measured using a laser Doppler vibrometer (UHF-120, Polytec GmBH, Waldbronn, Germany).
The exposure chamber shown in Fig. 1(a,b) was molded from polydimethylsiloxane cast in a 3D-printed mold, and designed to hold the SAW device at an angle to orient the sound radiated from it into the media containing the worms. The sound was made to propagate downwards and perpendicular to the chamber’s bottom surface, where the worms were placed, accomplished by mounting the SAW device at the Rayleigh angle \({\theta }_{R}={\sin }^{-1}({c}_{l}/{c}_{s})\approx 22^\circ \). When the SAW comes into contact with the liquid underneath it, the acoustic energy diffracts into the drop due to the mismatch between the sound velocity in the substrate, cs = 3990 m/s and the liquid, cl = 1485 m/s for water.
To test the hypothesis that cavitation is not responsible for the effects observed from the SAW exposure on the worms’ behavior, as shown in Fig. 4, three different media were chosen with significantly different viscosities and densities. The standard M9 buffer, less viscous polyvinyl alcohol (PVA) (5% wt), and highly viscous PVA (15% wt), and then exposed for 5 s at 1.5 W SAW power. This enables the testing of the hypothesis whether the cavitation is an injurious component of the SAW-driven ultrasound.
C. elegans model
C. elegans were cultured using standard conditions46, with the details of their culturing and use as follows. All chemicals and consumables were obtained from Fisher Scientific (Carlsbad, CA USA) unless otherwise noted. The N2 worm strain was used for all studies and purchased from the Caenorhabditis Genetics Center (CGC; University of Minnesota, Minneapolis MN). Nematodes were grown and maintained on nematode growth medium (NGM) agar plates containing a lawn of the bacterium Escherichia coli (OP50). NGM agar (3 g NaCl, 17 g bactoagar (BD–DFO140010), and 2.5 g bactopeptone (BD-211677) in 2 L) was autoclaved, post autoclaving, the flask was cooled in a 55 °C water bath and 1 mL of 1 M CaCl2, 1 mL of 5 mg/ml cholesterol in ethanol, 1 mL of 1 M MgSO4, and 25 mL of 1 M KPO4 buffer were added to the cooled agar. The agar was then poured onto plates and the plates were dried at room temperature overnight prior to seeding with OP50. The OP50 mixture was cultured overnight. After measuring out 100 mL of sterile Miller’s LB Broth (Corning), one colony of OP50 (CGC) was added to the flask. The culture was left to grow overnight at 37 °C in an incubator with constant shaking. 50–250 μL of the OP50 culture was added to the center of the dried plates. After seeding the plates with OP50, the plates were again dried overnight at room temperature prior to storage at 4 °C until use.
Age synchronization of worms was achieved via bleaching. Worms were allowed to grow until the adult stage. Gravid worms were recovered by washing plates with M9 buffer (3 g KH2PO4, 5.8 g Na2HPO4, 0.5 g NaCl, and 1 g 1 M NH4Cl to 2 L, autoclaved and then 2 mL of 1 M MgSO4 and 100 μL of 1 M CaCl2 was added) into 15 mL conical tubes. These worms were pelleted via centrifugation (1500 rpm for 2 minutes) and the supernatant was discarded. The worms were washed 2–3 more times with M9, repeating the centrifugation and supernatant removal steps. After the last washing step, a bleaching solution was added to each tube (3.5 mL H2O, 0.5 mL 5 M NaOH, and 1 mL sodium hypochlorite). The tubes were then placed on a gentle rocker for 7 minutes. Immediately after the 7 minute agitation step, the tubes were filled with 10 mL of M9 to stop the reaction and prevent any harm to the eggs. Next, a quick centrifugation step was performed (~1500 rpm for 1 min) and the supernatant was aspirated. The pellet was washed three more times with M9 buffer, and after the final wash, the pellet was resuspended in 200 μL of M9 and placed on seeded agar plates. The worms remained on the seeded agar plates for 48 hours at which time the worms were in the L4 stage and utilized for the experiments. The nematodes were kept in 20 °C incubator. All experiments, unless specifically noted, were run on nematodes age synchronized to L4.
Locomotion data collection
About 200–300 worms dispersed in \(100\,\mu \ell \) M9 solution70 were first placed at the bottom of the exposure chamber. An additional \(400\,\mu \ell \) M9 was added to the chamber to bring the liquid surface into contact with the SAW device (Fig. 1(a,b)). After exposure, C. elegans were rapidly removed using a pipette and dispensed drop by drop on to a polyethersulfone filter membrane (47 mm diameter, 0.22 μm pore size, Millipore, CA, USA) that was placed on the top of a vacuum filtration system comprised of a 150 ml filter bottle unit with 0.22 μm Millipore top filter (Millipore Express Plus 0.22 μm, Millipore, Temecula, CA). Once the liquid was removed, the membrane was removed and flipped over on a 6 cm agar plate seeded with OP50 E. coli. After a few seconds the membrane was removed, leaving most of the worms behind on the agar plate. The plate was immediately placed under a microscope (Leica KL200 LED, Leica Microsystems Inc., Buffalo Grove, IL, USA) and 1-min videos were then captured at 10 frames per second. After decompression and conversion to the AVI format, the videos were converted to 8-bit grayscale; background subtraction was performed using ImageJ and the resulting movie was thresholded to black-and-white using the automatic Otsu thresholding algorithm71. Next, the videos were analyzed using ImageJ (National Institutes of Health, Bethesda, Maryland) with the ImageJ plug-in wrMTrck72,73 to determine the average speed of movement of the C. elegans, reported in Fig. 1 as the average speed. The worms’ average movement speed was analyzed using a two-way ANOVA statistical method with \(\alpha =0.05\), where *p < 0.05. Normality of the data was assessed using the Shapiro-Wilk test with *p > α indicating the experimental data was normally distributed.
Associative memory assay
An associative learning and short-term memory assay similar to what was developed by A. Kauffman, et al.65 was used in this study. The memory assay involves three main steps: starving, short-term (1 hour) associative memory training, and a chemotaxis assay. The chemotaxis assay was performed on both naïve worms and non-naïve, trained worms immediately (0 hours), 1 hour, and 2 hours after training. More than 200 young adult wild-type worms were used per assay. Worms were first washed off of the high growth media (HGM) plates seeded with OP50 E. coli by pouring M9 buffer onto the plates. Then, the M9 buffer/worm mixture was transferred into a 15 mL conical tube where the worms were allowed to settle by gravity and the supernatant was removed by vacuum. Some of the worm population was used for the naïve chemotaxis assay while the remainder were kept in the conical tube in M9 buffer for 1 hour at room temperature to be starved. At the end of the starvation period, the supernatant M9 buffer was removed and worms were transferred to three training plates (>200 worms in each plate) seeded with OP50 E. coli and \(2\,\mu \ell \) of 10% butanone (in 95% ethanol) streaked on the inside of the lids of the plates. After 1 hour of associative learning (food-butanone association), the lids were washed 2–3 times with M9 buffer/water to remove the butanone. Trained worms were washed off of one of the plates for the 0 hour time point chemotaxis assay, and the two other plates were kept at room temperature for 1 and 2 hours to await their later chemotaxis assays.
For the chemotaxis assay, as shown in Fig. 3, a \(1\,\mu \ell \) paralyzing agent, 1 M NaN3, was first spotted at the control spots followed by \(1\,\mu \ell \) each of 95% ethanol and 10% butanone on top of the previously spotted NaN3. Next, the worms were transferred to the “origin” spot and the excess M9 buffer was removed using the twisted corner of a KimWipe. The total number of the worms was counted by capturing an image of the worms at the origin immediately after the worms were released onto the assay plate. Then, the closed-lid chemotaxis assay plate was incubated for 1 hour at room temperature. Finally, the worms were counted by taking images of the origin, ethanol, and butanone spots, and the massed chemotaxis index, \(CI=[({N}_{{butanone}})-({N}_{ethanol})]\)/\([({N}_{total})-({N}_{origin})]\), was computed from this information65. The chemotaxis index was calculated for naïve worms and worms after 0, 1, and 2 hours of associative memory training. Identical associative learning and chemotaxis (short-term associative memory) assays were conducted for the control (wild-type, unexposed C. elegans) and SAW-exposed worms. In the latter case, the sole difference in the process was the initial exposure of the naïve worms to SAW before the associative memory training and chemotaxis assay.
The massed chemotaxis index data was analyzed using a two-way ANOVA statistical method with \(\alpha =0.05\), where no significance (ns) refers to p > 0.05, *p < 0.05, **p < 0.01, and ***p < 0.0005. Specific changes in the definitions of the significance values are noted in the results where relevant. Normality of the data was assessed using the Shapiro-Wilk test with *p > α indicating the experimental data was normally distributed.
References
Alvarez, L. War veterans’ concussions are often overlooked. New York Times A1 (2008).
Hoge, C. W. et al. Mild traumatic brain injury in us soldiers returning from iraq. New England Journal of Medicine 358, 453–463 (2008).
Finkelstein, E. A., Corso, P. S. & Miller, T. R. Incidence and economic burden of injuries in the United States. (Oxford University Press, London UK, 2006).
Faul, M., Xu, L., Wald, M. M. & Coronado, V. Traumatic brain injury in the united states. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control (2010).
Antunes, M. & Biala, G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive Processing 13, 93–110 (2012).
DePalma, R. G., Burris, D. G., Champion, H. R. & Hodgson, M. J. Blast injuries. New England Journal of Medicine 352, 1335–1342 (2005).
Rosenfeld, J. V. et al. Blast-related traumatic brain injury. The Lancet Neurology 12, 882–893 (2013).
Heltemes, K. J., Holbrook, T. L., MacGregor, A. J. & Galarneau, M. R. Blast-related mild traumatic brain injury is associated with a decline in self-rated health amongst us military personnel. Injury 43, 1990–1995 (2012).
Ling, G., Bandak, F., Armonda, R., Grant, G. & Ecklund, J. Explosive blast neurotrauma. Journal of Neurotrauma 26, 815–825 (2009).
Fleminger, S. Head injury as a risk factor for alzheimer’s disease.(bnpa abstracts: Recovering from head injury). Journal of Neurology, Neurosurgery and Psychiatry 74, 832–833 (2003).
Johnson, V. E., Stewart, W. & Smith, D. H. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathology 22, 142–149 (2012).
Goldstein, L. E. et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Science Translational Medicine 4, 134ra60–134ra60 (2012).
Miller, G. Blast injuries linked to neurodegeneration in veterans. Science 336, 790–791 (2012).
Belanger, H. G., Kretzmer, T., Yoash-Gantz, R., Pickett, T. & Tupler, L. A. Cognitive sequelae of blast-related versus other mechanisms of brain trauma. Journal of the International Neuropsychological Society 15, 1–8 (2009).
Bombardier, C. H. et al. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. Jama 303, 1938–1945 (2010).
Bryan, C. J., Clemans, T. A., Hernandez, A. M. & Rudd, M. D. Loss of consciousness, depression, posttraumatic stress disorder, and suicide risk among deployed military personnel with mild traumatic brain injury. The Journal of Head Trauma Rehabilitation 28, 13–20 (2013).
Elder, G. A. et al. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. Journal of Neurotrauma 29, 2564–2575 (2012).
Luethcke, C. A., Bryan, C. J., Morrow, C. E. & Isler, W. C. Comparison of concussive symptoms, cognitive performance, and psychological symptoms between acute blast-versus nonblast-induced mild traumatic brain injury. Journal of the International Neuropsychological Society 17, 36–45 (2011).
Lau, K. M., Madden, E., Seal, K. & Maguen, S. Relationship of screen-based symptoms for mild traumatic brain injury and mental health problems in iraq and afghanistan veterans: Distinct or overlap** symptoms? Journal of Rehabilitation Research and Development 49, 1115 (2012).
Spikman, J. M., Timmerman, M. E., Milders, M. V., Veenstra, W. S. & van der Naalt, J. Social cognition impairments in relation to general cognitive deficits, injury severity, and prefrontal lesions in traumatic brain injury patients. Journal of Neurotrauma 29, 101–111 (2012).
Taber, K. H., Warden, D. L. & Hurley, R. A. Blast-related traumatic brain injury: what is known? The Journal of Neuropsychiatry and Clinical Neurosciences (2006).
Inglese, M. et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. Journal of Neurosurgery 103, 298–303 (2005).
Risdall, J. E. & Menon, D. K. Traumatic brain injury. Philosophical Transactions of the Royal Society of London B: Biological Sciences 366, 241–250 (2011).
**ong, Y., Mahmood, A. & Chopp, M. Animal models of traumatic brain injury. Nature Reviews Neuroscience 14, 128–142 (2013).
Nakagawa, A. et al. Mechanisms of primary blast-induced traumatic brain injury: insights from shock-wave research. Journal of Neurotrauma 28, 1101–1119 (2011).
Elder, G. A. & Cristian, A. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine 76, 111–118 (2009).
Chen, Y. C., Smith, D. H. & Meaney, D. F. In-vitro approaches for studying blast-induced traumatic brain injury. Journal of Neurotrauma 26, 861–876 (2009).
Gyorgy, A. et al. Time-dependent changes in serum biomarker levels after blast traumatic brain injury. Journal of Neurotrauma 28, 1121–1126 (2011).
Svetlov, S. I. et al. Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury. Journal of Neurotrauma 26, 913–921 (2009).
Tate, C. M. et al. Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: a breacher pilot study. Journal of Neurotrauma 30, 1620–1630 (2013).
Chen, X.-H., Johnson, V. E., Uryu, K., Trojanowski, J. Q. & Smith, D. H. A lack of amyloid β plaques despite persistent accumulation of amyloid β in axons of long-term survivors of traumatic brain injury. Brain Pathology 19, 214–223 (2009).
Johnson, V. E., Stewart, W. & Smith, D. H. Axonal pathology in traumatic brain injury. Experimental Neurology 246, 35–43 (2013).
Mendez, M. F. et al. Mild traumatic brain injury from primary blast vs. blunt forces: post-concussion consequences and functional neuroimaging. NeuroRehabilitation 32, 397–407 (2013).
Cernak, I. & Noble-Haeusslein, L. J. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. Journal of Cerebral Blood Flow & Metabolism 30, 255–266 (2010).
Cernak, I. Animal models of head trauma. NeuroRx 2, 410–422 (2005).
Kazanis, I. Cns injury research; reviewing the last decade: methodological errors and a proposal for a new strategy. Brain Research Reviews 50, 377–386 (2005).
LaPlaca, M., Simon, C., Prado, G. & Cullen, D. Cns injury biomechanics and experimental models. Progress in Brain Research 161, 13–26 (2007).
Manvelyan, H. Contemporary experimental models of traumatic brain injury. Georgian Medical News 13–16 (2006).
Morales, D. et al. Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience 136, 971–989 (2005).
Potts, M. B., Adwanikar, H. & Noble-Haeusslein, L. J. Models of traumatic cerebellar injury. The Cerebellum 8, 211–221 (2009).
Weber, J. T. Experimental models of repetitive brain injuries. Progress in Brain Research 161, 253–261 (2007).
Marklund, N., Bakshi, A., Castelbuono, D. J., Conte, V. & McIntosh, T. K. Evaluation of pharmacological treatment strategies in traumatic brain injury. Current Pharmaceutical Design 12, 1645–1680 (2006).
Gruber, J. et al. Caenorhabditis elegans: what we can and cannot learn from aging worms. Antioxidants & Redox Signaling 23, 256–279 (2015).
Leung, M. C. et al. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicological Sciences 106, 5–28 (2008).
Kaletta, T. & Hengartner, M. O. Finding function in novel targets: C. elegans as a model organism. Nature Reviews: Drug Discovery 5, 387 (2006).
Brenner, S. The genetics of caenorhabditis elegans. Genetics 77, 71–94 (1974).
Culetto, E. & Sattelle, D. B. A role for caenorhabditis elegans in understanding the function and interactions of human disease genes. Human Molecular Genetics 9, 869–877 (2000).
Hobert, O. The neuronal genome of caenorhabditis elegans. In The C. elegans Research Community (ed.) WormBook (WormBook).
Watts, D. J. & Strogatz, S. H. Collective dynamics of’small-world’networks. Nature 393, 440 (1998).
Hope, I. A. C. elegans: A Practical Approach. (Oxford University Press, London UK, 1999).
Lund, J. et al. Transcriptional profile of aging in c. elegans. Current Biology 12, 1566–1573 (2002).
Angstman, N. B., Kiessling, M. C., Frank, H.-G. & Schmitz, C. High interindividual variability in dose-dependent reduction in speed of movement after exposing C. elegans to shock waves. Frontiers in Behavioral Neuroscience 9 (2015).
Ibsen, S., Tong, A., Schutt, C., Esener, S. & Chalasani, S. H. Sonogenetics is a non-invasive approach to activating neurons in caenorhabditis elegans. Nature Communications 6 (2015).
Zhou, W. et al. Ultrasound neuro-modulation chip: activation of sensory neurons in caenorhabditis elegans by surface acoustic waves. Lab on a Chip 17, 1725–1731 (2017).
Yuan, J., Zhou, J., Raizen, D. M. & Bau, H. H. High-throughput, motility-based sorter for microswimmers such as C. elegans. Lab on a Chip 15, 2790–2798 (2015).
San-Miguel, A. & Lu, H. Microfluidics as a tool for c. elegans research. In The C. elegans Research Community (ed.) WormBook (WormBook).
Ben-Yakar, A., Chronis, N. & Lu, H. Microfluidics for the analysis of behavior, nerve regeneration, and neural cell biology in c. elegans. Current Opinion in Neurobiology 19, 561–567 (2009).
Friend, J. & Yeo, L. Y. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Reviews of Modern Physics 83, 647 (2011).
Ding, X. et al. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proceedings of the National Academy of Sciences 109, 11105–11109 (2012).
Ahmed, D. et al. Rotational manipulation of single cells and organisms using acoustic waves. Nature Communications 7 (2016).
Connacher, W. et al. Micro/nano acoustofluidics: materials, phenomena, design, devices, and applications. Lab Chip 18, 1952–1996 (2018).
Collignon, S., Manor, O. & Friend, J. Improving and predicting fluid atomization via hysteresis-free thickness vibration of lithium niobate. Advanced Functional Materials 28 (2018).
Noltingk, B. E. & Neppiras, E. A. Cavitation produced by ultrasonics. Proceedings of the Physical Society. Section B 63, 674 (1950).
Dentry, M. B., Yeo, L. Y. & Friend, J. R. Frequency effects on the scale and behavior of acoustic streaming. Physical Review E 89, 013203 (2014).
Kauffman, A. et al. C. elegans positive butanone learning, short-term, and long-term associative memory assays. Journal of Visualized Experiments: JoVE 2490 (2011).
Delius, M. & Gambihler, S. Effect of shock waves on gallstones and materials. In Lithotripsy and related techniques for gallstone treatment. Proceedings of the 3rd International Symposium on Biliary Lithotripsy. St. Louis: Mosby Year Book, 27–33 (1991).
Schelling, G., Delius, M., Gschwender, M., Grafe, P. & Gambihler, S. Extracorporeal shock waves stimulate frog sciatic nerves indirectly via a cavitation-mediated mechanism. Biophysical Journal 66, 133–140 (1994).
Hayakawa, K., Takeda, S., Kawabe, K. & Shimura, T. Acoustic characteristics of pva gel. In Proceedings of the 1989 Ultrasonics Symposium, 969–972 (IEEE, 1989).
Baasch, T. et al. Acoustic compressibility of caenorhabditis elegans. Biophysical Journal, https://doi.org/10.1016/j.bpj.2018.08.048 (2018).
Porta-de-la Riva, M., Fontrodona, L., Villanueva, A. & Cerón, J. Basic caenorhabditis elegans methods: Synchronization and observation. Journal of Visualized Experiments: JoVE 4019 (2012).
Kurita, T., Otsu, N. & Abdelmalek, N. Maximum likelihood thresholding based on population mixture models. Pattern Recognition 25, 1231–1240 (1992).
MS Windows NT kernel description, http://www.phage.dk/plugins/wrmtrck.html (Accessed: 08-16-2018).
Nussbaum-Krammer, C. I., Neto, M. F., Brielmann, R. M., Pedersen, J. S. & Morimoto, R. I. Investigating the spreading and toxicity of prion-like proteins using the metazoan model organism c. elegans. Journal of visualized experiments: JoVE (2015).
Acknowledgements
The authors are grateful to the University of California and the NANO3 facility at UC San Diego for provision of funds and facilities in support of this work. Some C. elegans strains were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was performed in part at the San Diego Nanotechnology Infrastructure (SDNI) of UCSD, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (Grant ECCS–1542148). The authors are also grateful for the support of this work by NIH Grant T32-GM121318-01. The work presented here was generously supported in part by a research grant from the W.M. Keck Foundation.
Author information
Authors and Affiliations
Contributions
J.F. and H.P. conceived the experiment(s), M.M., M.D.M., J.S. and Y.K. conducted the experiments, M.M., J.S., Y.K., H.P. and J.F. analyzed the results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miansari, M., Mehta, M.D., Schilling, J.M. et al. Inducing Mild Traumatic Brain Injury in C. elegans via Cavitation-Free Surface Acoustic Wave-Driven Ultrasonic Irradiation. Sci Rep 9, 12775 (2019). https://doi.org/10.1038/s41598-019-47295-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47295-1
- Springer Nature Limited