Abstract
Invasive species that rapidly spread throughout novel distribution ranges are prime models to investigate climate-driven phenotypic diversification on a contemporary scale. Previous studies on adaptive diversification along latitudinal gradients in fish have mainly considered body size and reported either increased or decreased body size towards higher latitudes (i.e. Bergmann’s rule). Our study is the first to investigate phenotypic divergence in multiple traits, including sexually selected traits (size and shape of the male copulatory organ, the gonopodium) of invasive Gambusia affinis in China. We studied body size, life history traits and morphological variation across populations spanning 17 degrees of latitude and 16 degrees of longitude. Even though we found phenotypic variation along climatic gradients to be strongest in naturally selected traits, some sexually selected traits also showed systematic gradual divergence. For example, males from southern populations possessed wider gonopodia with increased armament. Generally, males and females diverged in response to different components of climatic gradients (latitudinal or longitudinal variation) and in different trait suites. We discuss that not only temperature regimes, but also indirect effects of increased resource and mate competition (as a function of different extrinsic overwinter mortality rates) alter the selective landscape along climatic gradients.
Similar content being viewed by others
Introduction
Environmental variation and adaptation along climatic gradients
Identifying the ecological factors driving phenotypic diversification along climatic gradients lies at the heart of research in biogeography and evolutionary ecology1,2,3. The multivariate variation of ecological conditions along climatic gradients—especially in mean annual temperature4 and (daily or seasonal) temperature fluctuation5,6, but also in predation7,8,9,10 and other biotic factors11,12—creates divergent selective regimes that affect phenotypic traits directly related to fitness, including physiological, morphological, reproductive, and behavioral traits13,14,15,16. Studies over large geographic scales (in terms of longitudinal, latitudinal, and/or altitudinal variation) are likely to capture systematic variation not only in abiotic, but also in biotic selection factors and provide important insights into the mechanisms underlying the observed phenotypic divergence.
Adaptive phenotypic divergence along extensive climatic gradients has been reported for several taxa, including insects17, birds18 and mammals19. Phenotypic variation along latitudinal gradients received most scientific attention20,21,22. Variation in temperature regimes, precipitation, photo- and vegetation periods, to mention but some important abiotic factors, bring about an array of correlated changes in biotic selection factors (e.g., regarding species richness, primary production, and resource availability)23,24. Latitudinal variation in body size has been examined thoroughly25,26,27. Body size is linked to fitness as it not only influences physiological performance in contrasting thermal environments (with passive heat loss being reduced as the body volume-to-surface ratio increases28), but can also affect traits like anti-predator behavior (e.g., through altered maneuverability)29,30,31. In this context, Bergmann’s rule arguably represents the most widely known ecogeographic rule. It states that within a given taxonomic group of endotherms (populations, species, or higher taxonomic levels), larger body size would be predicted in colder environments, i.e., towards higher latitudes32,33. Bergmann’s rule has received extensive support from studies on different endotherms34,35, while the evidence for ectotherms is controversial36,37,38,39. For instance, revisiting n = 703 angling records from populations of 29 North American freshwater fishes, Rypel40 demonstrated that only 38% of species follow Bergmann’s rule, while 34% showed the reversed pattern, and the remaining 28% showed no intraspecific body size variation related to latitude. Another widely known ecogeographic law is Allen’s rule41, which states that the body extremities of endotherms that live under cold climatic conditions (i.e., at higher latitudes) are smaller than those of related taxa living at lower latitudes. Just like an increased body mass (i.e., Bergmann’s rule), shorter body appendages are thought to help increase the body volume-to-surface ratio, thereby minimizing thermal energy loss in cold environments42,43,44.
Does sexual selection contribute to phenotypic divergence along climatic gradients?
Studies throughout the Animal Kingdom reported that body size is not only under natural but also sexual selection, e.g., via mate competition (intrasexual selection) or female mate choice (intersexual selection)45,46,47,48. More generally speaking, phenotypic traits typically considered to show latitudinal divergence in response to natural selection could also diverge—at least in part—through different forms of sexual selection. Regarding body size, this could be true especially for ectotherms, for which the above-mentioned form of natural selection from climatic variation does not readily apply49. Certain forms of sexual selection could be stronger at lower latitudes, where population densities and mate encounter rates can be higher50,51. However, the role played by sexual selection during phenotypic diversification along latitudinal gradients is generally not well understood.
Our present study provides novel insights into the potential contributions of both natural and sexual selection in driving phenotypic variation in invasive Western mosquitofish (Gambusia affinis) along climatic (latitudinal and longitudinal) gradients in the species’ invasive distribution range in China52. However, our study design was not suitable to tease apart the relative influences of natural and sexual selection on body size, and so investigating this question will be reserved to future studies. Still, we provide indirect evidence that both forms of selection are involved in phenotypic diversification along climatic gradients. Specifically, we demonstrate divergence in various phenotypic traits (male and female body size, body shape, and life histories), while including traits that are known to be under strong sexual selection.
Multivariate phenotypic trait divergence in invasive mosquitofish
In an attempt to control mosquito-borne diseases, Western (G. affinis) and Eastern mosquitofish (G. holbrooki) have been introduced to at least 40 countries worldwide53,54,55, including the introduction of G. affinis to large parts of mainland China52,56. A recent study57 demonstrated that latitudinal body size variation of the closely related G. holbrooki in its native range in the Eastern USA is in support of Bergmann’s rule. Considering various ecological factors covarying with climate along the examined stretch of >14 degrees of latitude (such as the thermal regime, local population densities, and habitat productivity), a model selection approach identified the thermal regime as the main selection force driving the pattern of increasing body size with increasing latitude. Reproductive strategies showed r-selected life-history patterns at high latitudes [with high reproductive allocation (RA) and numerous small offspring], which could be owing to higher extrinsic mortality rates. On the other hand, other traits, like body condition and body shape, appear to diverge as a function of habitat productivity and population density. However, in another study49, G. affinis from 27 populations spanning nine degrees of latitude in North America showed a suggestive trend contradicting Bergmann’s rule. Finally, Stockwell and Vinyard58 studied life-history variation of four newly established (invasive) G. affinis populations and found small body size, early maturity, low fat reserves and small embryos in female G. affinis from thermally unstable environments.
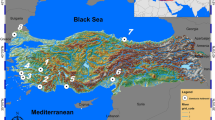
In this study, we collected invasive mosquitofish along 17 degrees of latitude and 16 degrees of longitude in mainland China (Fig. 1a). Based on existing theories and recent studies, we tested the following predictions:
-
(1)
Body size: Following a previous study in the congener G. holbrooki, we predicted that invasive G. affinis in China have larger body size at higher latitudes, partly because bigger individuals have an advantage in terms of greater overwintering survival in harsh environments59,60. On the other hand, body size could also show a pattern contradicting Bergmann’s rule49: life-history theory predicts that high adult mortality in fluctuating environments (i.e., higher latitudes, and continental/inland sites61) selects for early maturity and thus, small adult body size62. Moreover, lower resource availability in colder environments impairs growth rates63. We refrain from formulating predictions for body size evolution by sexual selection, but we will tentatively discuss our results in light of the insights into the general involvement of sexual selection in driving trait divergence, as obtained from our analyses of the size and shape of the distal part of the male intromittant organ, called gonopodium64,65.
-
(2)
Life-history traits: G. affinis at higher latitudes are likely to experience high overwinter mortality66,67. Other environmental factors, such as fluctuating productivity68, should increase (unpredictable) mortality rates. Based on life-history theory62,69, we predicted G. affinis females to produce more but smaller offspring at higher latitudes, and to have a higher total investment into reproduction. More stable and benign conditions at lower latitudes likely result in higher survival and continuously higher population densities. Increased intraspecific competition should favor the production of fewer but bigger offspring, which are more competitive62,70.
-
(3)
Morphology: Since female body shape is tightly linked to life-history traits71,72,73, we predicted that divergence in female body shape largely follows patterns predicted for life-history divergence. Populations at higher latitudes—characterized by higher reproductive effort—should have enlarged abdomens to harbor larger broods, more anteriorly positioned pectoral fins and relatively smaller heads than more southern populations. By contrast, males are unlikely to show a similar degree of morphological divergence mirroring life-history divergence.
-
(4)
Gonopodium morphology: Populations from lower latitudes and from more coastal areas (which are preferred by mosquitofish53) likely experience more stable and benign environmental conditions. Low overwinter mortality should result in higher population densities and heightened intrasexual competition amongst males. Also, mosquitofish females prefer males with longer gonopodia64, and females are more likely to exert mate choice when population densities are high, as they have more opportunities to choose (even though this effect may be weakened by coercive mating74,75,76,77). This could result in elongated gonopodia (via female choice) and more rigid distal gonopodium tips (a trait that is beneficial to achieve coercive copulations65) towards lower latitudes and in coastal areas.
Sampling sites and morphological landmarks. (a) Ten sampling sites across China from which adult G. affinis were collected; city name codes can be found in Table 3. The map was generated using DAVI-GIS v 7.5.0 (http://www.diva-gis.org/). (b) Male (above) and female G. affinis (below) collected in Hangzhou in April 2016. Dots and numbers indicate the 13 landmarks used for morphometric analyses and two additional landmarks (14 and 15) used for the ‘unbending’ procedure in our Procrustes analyses. (c) Exemplary micrograph of a gonopodium showing the 51 morphometric landmarks. Nomenclature of fin rays (3, 4a, 4p, 5a) follows Rosen and Gordon108. (d–f) Exemplary photos of our sampling sites in Ankang, Chaozhou and Nan**g, respectively.
Results
Population genetic analyses
We conducted population genetic analyses based on 15 nuclear microsatellites78,79,80. This part of our study served not only as a validation of species identity56, but also tested for ‘unusual’ patterns of population genetic structure (suggesting recent translocations or multiple introductions)—important background information for the interpretation of our data on phenotypic divergence. Standard population genetic parameters for each population can be found in Table S1. We found varying degrees of genetic differentiation between populations, ranging from virtual panmixis (FST = 0.038, between Nan**g and Hangzhou) to moderate genetic differentiation (FST = 0.268, between **-stone-like fashion, or at least some degree of ongoing gene-flow between populations.
Population genetic analyses of invasive G. affinis in mainland China. (a) Genetic structure among populations (see Table 1 for population codes). Individual assignment to two genetically distinct clusters using STRUCTURE168. Likelihood of assignment for each individual is shown as a vertical bar. (b) Bayesian inference of the number of genetically distinct clusters (K) among the 10 sampled populations using ΔK169. (c) Principal coordinate analysis (PCoA) showing genetic differentiation between populations according to the first two axes. Percent variance explained is given in parentheses. Triangles indicate positive values of the third axis (12.22% variance explained), while squares indicate negative values. Colors signify assignment to K = 2 genetically distinct clusters in STRUCTURE. (d) Neighbor-joining tree based on genetic distances (Nei’s DA). Numbers at nodes indicate bootstrap support; only values >70 are presented. (e) Correlation between genetic distance and geographic distance. A Mantel test on log-transformed pairwise genetic distances (FST-values) detected a significant effect of log-transformed geographic distances (Z = −103.73, r = 0.37, one-sided P = 0.014).
According to bottleneck analyses under three microsatellite evolution models, most of the populations underwent genetic bottlenecks in the recent past—especially the populations from Ankang, ** life-history trait divergence along climatic gradients. Sperm competition intensifies as a function of lower overwinter mortality under benign (southern) conditions118,119. A higher GSI may allow for increased sperm production under these circumstances120,121. However, further studies are required to fully elucidate the impact of population densities on the observed life-history divergence. Likewise, female poeciliids typically produce fewer and bigger offspring that are more competitive under fierce resource competition70,121,122,123. Decreased fat content towards southern populations in both sexes could thus reflect a trade-off between reproductive investment and investment into somatic maintenance in the face of strong resource competition117. We are lacking a clear explanation with respect to the observed divergence of somatic lean weight, but increased lean weight could reflect an adaptation to enhance growth and reproduction during the shorter growing seasons in northern latitudes57.
Females (from northern populations) and males showing increased fat content in inland populations may be indicative of relaxed resource competition towards inland sites. The pattern observed in females from southern sites, however, matches predation-driven patterns described for other poeciliids, with lower fat content, and more but smaller offspring being produced in inland populations14,124,125. Large body size in poeciliids can be accompanied by an increased risk of falling victim to predation126,127, and avian predation exerts strong selection on body size at least in natural G. affinis populations128. However, we are currently lacking empirical data on potential variation in avian predation along the climatic gradients examined here.
Body shape and size variation
We hypothesized that body shape divergence would primarily follow patterns observed for life-history diversification, with enlarged abdominal cavities, anteriorly positioned pectoral fins and smaller heads at higher latitudes. We found patterns of divergence to be seemingly congruent with our predictions, but notably, the pattern was more clear-cut for males than for females. This sheds doubt on our initial hypothesis that body shape would evolve as an indirect consequence of life-history divergence, in which case females should show the strongest body shape divergence. We further predicted increased body size at higher latitudes and inland sites because large-bodied individuals have a higher survival rate in harsh and fluctuating environments. Our predictions were met along the latitudinal gradient (climatic PC1) but reversed along the longitudinal gradient (PC2), and the pattern was only observed in males.
Previous studies on freshwater fishes identified several agents of natural selection to affect body shape and size, including flow regime129, resource availability130,131, and predation risk14,89,90,91,132. We argue that variation in both body shape and size of male G. affinis in our study system is primarily driven by temperature regimes. Low overwinter temperatures at higher latitudes select for endurable individuals with larger body size133 and larger fat reserves134. Larger fat stores could indeed explain enlarged abdominal cavities of male G. affinis from northern populations. Mosquitofish males are much smaller, on average, than females135, and so males may be under stronger selection for increased body size (and more compact shape) at higher latitudes. Moreover, we suggested increased intrasexual competition in southern populations, but also higher predation pressure in the south could translate into more forced copulations10,127. Enlarged caudal regions, smaller heads, and a more elongated body are traits that improve unsteady swimming87,136, which is also important during coercive mating. Moreover, small-bodied males can approach females in the blind portion of their visual field, thus preventing females from fleeing, and have a better maneuverability than large-bodied ones137,138,139.
Predation selects for early maturity and smaller adult body size in numerous poeciliid species140 like Poecilia reticulata14, P. vivipara141, Brachyrhaphis episcopi142 or Phalloceros harpagos143. The smaller body size of males from inland populations could again point towards a role for increased predation risk along the longitudinal gradient (see above), for which we do not currently have empirical evidence at hand. As poeciliid females tend to show a preference for large-bodied males64,175. Following the methods described in Chapuis and Estoup176, we used FreeNA to calculate unbiased FST-values between populations while accounting for potential null alleles. To estimate the degree of isolation-by-distance among populations, we performed a Mantel test with pairwise FST-values (calculated with FreeNA using the ENA correction) and linear geographic distances (obtained from Google Earth) using IBDWS v 3.23 (http://ibdws.sdsu.edu/ibdws/distances.html). We tested for evidence of genetic bottlenecks in each population separately using Bottleneck v 1.2.02177. We used Wilcoxon signed-rank tests to identify recently bottlenecked populations by comparing observed and expected numbers of loci with heterozygosity excess under three mutation models, the infinite allele model (IAM), stepwise mutation model (SMM), and two-phase model (TPM), respectively, as recommended by Luikart and Cornuet178.
We used STRUCTURE v 2.3.4179 to calculate individual assignment probabilities (Q-values) to varying numbers of genetically distinct clusters (K). For each value of K = 1–10, ten iterations were run using the admixture model with a burn-in period of 250,000 generations, followed by a sampling phase of 750,000 iterations. We detected the uppermost level of population differentiation with the method presented by Evanno et al.180 using the web-based tool STRUCTURE HARVESTER v 0.6.94. Furthermore, we calculated genetic distances181 (Nei’s DA) using Populations v 1.2.32 (http://bioinformatics.org/project/?group_id=84) and visualized a neighbor-joining tree using TreeView v 1.6.6182 (http://taxonomy.zoology.gla.ac.uk/rod/rod.html). The bootstrap** procedure implemented in Phylip v 3.695 (http://evolution.genetics.washington.edu/phylip.html) was used to evaluate the significance of tree nodes (based on allele frequencies, with 1,000 bootstrap replicates). Moreover, we analyzed genetic structure among populations by means of a principal coordinate analysis (PCoA) based on pairwise Nei’s using GenAlEx v 6.503183,184.
Body size and life histories
We included n = 184 males and n = 191 females in the analysis of life-history traits (18 to 52 individuals per population). We measured standard lengths (SL) of each individual using digital calipers (accurate to the closest 0.01 mm). Maturity was assessed by inspecting the opened body cavity for develo** ova (females) or mature testes (males). Afterwards, we removed all reproductive tissues and all develo** embryos. We determined the stage of development and number of embryos (fecundity) for each female185. Somatic tissues, along with gonads or embryos, were then dried at 55 °C for 24 hours. To assess female and embryo body condition, dried samples were washed for at least six hours in petroleum ether to extract non-structural body fat and were then re-dried and re-weighed.
We thus assessed standard length (SL [mm]), somatic dry weight [mg], somatic lean weight [mg], and fat content [%] for both sexes, the GSI [%] for males, and fecundity (number of develo** embryos), RA [%], embryo lean weight [mg], as well as embryo fat content [%] in case of females. Reproductive effort (i.e. GSI for males and RA for females) was calculated by dividing gonad dry weight (plus embryo dry weight in the case of females) by the sum of gonad (plus embryo) and somatic dry weights. We log10-transformed SL, somatic dry weight, and somatic lean weight, arcsine (square root)-transformed somatic fat content, GSI, RA and embryo fat content, and square root-transformed fecundity. Z-transformation was subsequently applied to all data to obtain unit-free data with equal variance.
To assess the extent of divergence along climatic gradients, we used MANCOVA using the two climate-related PCs (see above) as covariates. Throughout this study, we also included the interaction term of both climate-related covariates but removed it from the final models if not significant. In male life-history analyses, we further included SL as a covariate, while SL and the embryos’ developmental stage served as additional covariates in the case of females. We ran post-hoc ANCOVAs of the exact same structure as the final retained MANCOVA model, to identify the source(s) of variation in case of significant model terms. To evaluate the relative importance of each term, we estimated effect sizes by calculating Wilk’s partial eta squared (ηp2)85. Furthermore, we report relative variance explained by model terms as the partial variance explained for a given term divided by the maximum partial variance in that model.
Generally, to visualize significant interaction effects, we split the data into inland (climate-related PC2 ≥ median) and coastal populations (PC2 < median) and depict variation along PC1 (latitudinal variation) for both cohorts. The alternative way of depicting variation along PC2, while splitting the data based on median values of PC1, is shown in Supplementary Figs S1 and S2.
Geometric morphometrics
We included n = 191 males and n = 211 females in the analysis of body shape divergence (17 to 57 individuals per population). We took lateral photographs of alcohol-preserved individuals (left body side) that were placed in a paraffin-coated petri-dish alongside a piece of laminated scale grid paper using a Canon EOS 760D single lens reflex camera (CANON INC., Ota-Ku, Japan). Photos were loaded into tps format using tpsUtil software186, after which we digitized 13 landmarks and measured gonopodium length (in the case of males) using tpsDig2 v 2.26187 (Fig. 1b). Landmarks provided adequate coverage of the lateral body contour of mosquitofish82,188. To correct for bending effects, we applied the ‘Unbend specimens’ function in tpsUtil using landmarks 1 and 6, as well as two additional landmarks (14 and 15) that were removed from the final analysis (Fig. 1b). We then applied a full Procrustes fit procedure using the software MorphoJ188. This procedure superimposes shape coordinates in a linear tangent space and automatically excludes variation that is not caused by true shape-variation (i.e. translation, scaling and rotation effects). After extracting shape information, a factor reduction procedure was performed in MorphoJ to reduce data dimensionality. We retained ten morphology-related PCs for both males and females, which accounted for 88.31% (males) and 88.81% (females) of the total morphological variance, respectively.
Our main analytical MANCOVA used morphology-related PCs as dependent variables and log10-transformed centroid size along with the two climate-related PCs (see above) as covariates. Again, post-hoc ANCOVAs on single PCs were conducted as described above. Significant effects for PCs that explained only a small percentage of shape variation (≤6.42%) can be found in Supplementary Figs S3–5.
Gonopodium morphology and length
We assessed morphological information on gonopodium tip structures of n = 183 males from eight populations (12 to 35 individuals per population) as we missed to assess gonopodium morphology in the **amen and Nan**g populations. Because the male gonopodium is a delicate organ, we cut the entire gonopodium and photographed the distal tip laterally (left side) at 100× magnification using an Optec B 302 microscope equipped with an Optec TP510 CCD camera (both from Optec Instrument Co. ltd., Chongqing, China). We used 51 homologous landmarks described by Heinen-Kay and Langerhans54 to capture morphological variation (Fig. 1c). Using similar Procrustes analyses and PCA procedures as described above, we obtained nine PCs that cumulatively explained 89.93% of the total variance. We conducted MANCOVAs using morphology-related PCs as dependent variables, while including log10-transformed centroid size, total gonopodium length (determined during the assessment of body shape information, see above) and both climate-related PCs as covariates. We performed post-hoc ANCOVAs to determine the source(s) of variation in case of significant model terms. We subjected the data on gonopodium lengths (from all 10 populations) to an ANCOVA, using standard length and the two climatic PCs as covariates.
Data availability
The datasets generated and/or analyzed for the current study are available from the corresponding author on reasonable request.
References
Lomolino, M. V. Elevation gradients of species-density: historical and prospective views. Global Ecol. Biogeogr. 10, 3–13 (2001).
Mittelbach, G. et al. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (2007).
Pearson, R. G. & Dawson, T. P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 12, 361–371 (2003).
Royer, D. L., Meyerson, L. A., Robertson, K. M. & Adams, J. M. Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum. PLoS One 4, e7653 (2009).
Robin, J. P. & Denis, V. Squid stock fluctuations and water temperature: temporal analysis of English Channel Loliginidae. J. Appl. Ecol. 36, 101–110 (1999).
Uvarov, A. V., Tiunov, A. V. & Scheu, S. Effects of seasonal and diurnal temperature fluctuations on population dynamics of two epigeic earthworm species in forest soil. Soil Biol. Biochem. 43, 559–570 (2011).
Rieger, J. F., Binckley, C. A. & Resetarits, W. J. Larval performance and oviposition site preference along a predation gradient. Ecology 85, 2094–2099 (2004).
Jeanne, R. L. A latitudinal gradient in rates of ant predation. Ecology 60, 1211–1224 (1979).
Peckarsky, B. L., Horn, S. C. & Statzner, B. Stonefly predation along a hydraulic gradient: a field test of the harsh–benign hypothesis. Freshw. Biol. 24, 181–191 (2010).
Reznick, D., Butler, M. J. & Rodd, H. Life-history evolution in guppies. VII. The comparative ecology of high- and low-predation environments. Am. Nat. 157, 126–140 (2001).
Menge, B. A. & Sutherland, J. P. Species diversity gradients: synthesis of the roles of predation, competition, and temporal heterogeneity. Am. Nat. 110, 351–369 (1976).
Goldberg, D. E., Rajaniemi, T., Gurevitch, J. & Stewart-Oaten, A. Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology 80, 1118–1131 (1999).
Blumenshine, S. C., Lodge, D. M. & Hodgson, J. R. Gradient of fish predation alters body size distributions of lake benthos. Ecology 81, 374–386 (2000).
Reznick, D. & Endler, J. A. The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36, 160–177 (1982).
Heinen-Kay, J. L., Noel, H. G., Layman, C. A. & Langerhans, R. B. Human-caused habitat fragmentation can drive rapid divergence of male genitalia. Evol. Appl. 7, 1252–1267 (2014).
Culumber, Z. W., Shepard, D. B., Coleman, S. W., Rosenthal, G. G. & Tobler, M. Physiological adaptation along environmental gradients and replicated hybrid zone structure in swordtails (Teleostei: **phophorus). J. Evol. Biol. 25, 1800–1814 (2012).
Harris, R., Mcquillan, P. & Hughes, L. Patterns in body size and melanism along a latitudinal cline in the wingless grasshopper. Phaulacridium vittatum. J. Biogeogr. 39, 1450–1461 (2012).
Phillimore, A. B. et al. Biogeographical basis of recent phenotypic divergence among birds: a global study of subspecies richness. Evolution 61, 942–957 (2007).
Weir, J. T. & Schluter, D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315, 1574–1576 (2007).
Vandewoestijne, S. & Van Dyck, H. Population genetic differences along a latitudinal cline between original and recently colonized habitat in a butterfly. PLoS One 5, e13810, https://doi.org/10.1371/journal.pone.0013810 (2010).
Alho, J. S. et al. Allen’s rule revisited: quantitative genetics of extremity length in the common frog along a latitudinal gradient. J. Evol. Biol. 24, 59–70 (2011).
Ebert, T. A. et al. Growth and mortality of red sea urchins Strongylocentrotus franciscanus across a latitudinal gradient. Mar. Ecol. Prog. Ser. 190, 189–209 (1999).
Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 8, 224–239 (2005).
Willig, M. R., Kaufman, D. M. & Stevens, R. D. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol Syst. 34, 273–309 (2003).
Loeschcke, V., Bundgaard, J. & Barker, J. S. F. Variation in body size and life history traits in Drosophila aldrichi and D. buzzatii from a latitudinal cline in eastern Australia. Heredity 85, 423–433 (2000).
Sand, H., Cederlund, G. & Danell, K. Geographical and latitudinal variation in growth patterns and adult body size of swedish moose (Alces alces). Oecologia 102, 433–442 (1995).
Olson, V. A. et al. Global biogeography and ecology of body size in birds. Ecol. Lett. 12, 249–259 (2009).
Vogel, S. Size and shape In Life’s devices: the physical world of animals and plants. pp 38–59 Princeton University Press (1988).
Anderson, V. R. & Alisauskas, R. T. Egg size, body size, locomotion, and feeding performance in captive King Eider ducklings. Condor 103, 195–199 (2001).
Nauen, J. C. & Shadwick, R. E. The scaling of acceleratory aquatic locomotion: body size and tail-flip performance of the California spiny lobster Panulirus interruptus. J. Exp. Biol. 202, 3181–3193 (1999).
Reichle, D. Relation of body size to food intake, oxygen consumption, and trace element metabolism in forest floor arthropods. Ecology 49, 538–542 (1968).
Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Tiere zu ihrer Grösse. Göttinger Studien 1, 595–708 (1847).
Salewski, V. & Watt, C. Bergmann’s rule: a biophysiological rule examined in birds. Oikos 126, 161–172 (2017).
Meiri, S. & Dayan, T. On the validity of Bergmann’s rule. J. Biogeogr. 30, 331–351 (2003).
Kyle, G. A., Mark, C. T. & Alan de, Q. Is Bergmann’s rule valid for mammals? Am. Nat. 156, 390–415 (2000).
Adams, D. C. & Church, J. O. Amphibians do not follow Bergmann’s rule. Evolution 62, 413–420 (2010).
Mousseau, T. A. Ectotherms follow the converse of Bergmann’s rule. Evolution 51, 630–632 (1997).
Zamora-Camacho, F. J., Reguera, S. & Moreno-Rueda, G. Bergmann’s rule rules body size in an ectotherm: heat conservation in a lizard along a 2200-metre elevational gradient. J. Evol. Biol. 27, 2820–2828 (2014).
Osorio-Canadas, S. et al. Body size phenology in a regional bee fauna: a temporal extension of Bergmann’s rule. Ecol. Lett. 19, 1395–1402 (2016).
Rypel, A. L. The cold-water connection: Bergmann’s rule in north American freshwater fishes. Am. Nat. 183, 147–156 (2014).
Allen, J. A. The influence of physical conditions in the genesis of species. Radic. Rev. 1, 108–140 (1877).
Symonds, M. R. & Tattersall, G. J. Geographical variation in bill size across bird species provides evidence for Allen’s rule. Am. Nat. 176, 188–197 (2010).
Griffing, J. P. Body measurements of black-tailed jackrabbits of southeastern New Mexico with implications of Allen’s rule. J. Mammal. 55, 674–678 (1974).
Shelomi, M. & Zeuss, D. Bergmann’s and Allen’s rules in native European and Mediterranean Phasmatodea. Front. Ecol. Evol. 5, https://doi.org/10.3389/fevo.2017.00025 (2017).
Schluter, D. & Smith, J. Natural selection on beak and body size in the song sparrow. Evolution 40, 221–231 (1986).
Searcy, W. A. Sexual selection and body size in male red-winged blackbirds. Evolution 33, 649–661 (1979).
Wilbur, H. M., Rubenstein, D. I. & Fairchild, L. Sexual selection in toads: the roles of female choice and male body size. Evolution 32, 264–270 (1978).
Head, M. L., Kahn, A. T., Henshaw, J. M., Keogh, J. S. & Jennions, M. D. Sexual selection on male body size, genital length and heterozygosity: consistency across habitats and social settings. J. Anim. Ecol. 86, 1458–1658 (2017).
Belk, M. C. & Houston, D. D. Bergmann’s rule in ectotherms: a test using freshwater fishes. Am. Nat. 160, 803–808 (2002).
Arnqvist, G. Spatial variation in selective regimes: sexual selection in the water strider. Gerris odontogaster. Evolution 46, 914–929 (1992).
Kokko, H., Klug, H. & Jennions, M. D. Unifying cornerstones of sexual selection: operational sex ratio, Bateman gradient and the scope for competitive investment. Ecol. Lett. 15, 1340–1351 (2012).
Li Z. Y. & **e Y. Invasive species in China. China Forestry Publishing House, Bei**g 88 pp. (2002).
Pyke, G. H. A review of the biology of Gambusia affinis and G. holbrooki. Rev. Fish Biol. Fisher. 15, 339–365 (2005).
Pyke, G. H. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu. Rev. Ecol. Evol. Syst. 39, 171–191 (2008).
Azevedo-Santos, V. M., Vitule, J. R. S., Pelicice, F. M., Garcia-Berthou, E. & Simberloff, D. Nonnative fish to control Aedes mosquitoes: a controversial, harmful tool. BioScience 67, 83–89 (2017).
Gao, J. C., Ouyang, X., Chen, B. J., Jourdan, J. & Plath, M. Molecular and morphometric evidence for the widespread introduction of western mosquitofish (Gambusia affinis) into freshwaters of mainland China. BioInvas. Rec. 6, 281–289 (2017).
Riesch, R. et al. Thermal regime drives a latitudinal gradient in morphology and life history in a livebearing fish. Biol. J. Linn. Soc. (in press).
Stockwell, C. A. & Vinyard, G. L. Life history variation in recently established populations of Western mosquitofish (Gambusia affinis). West. N. Am. Nat. 60, 273–280 (2000).
Daniels, G. L. & Felley, J. D. Life-history and foods of Gambusia affinis in two waterways of southwestern Louisiana. Southw. Nat. 37, 157–165 (1992).
Pangle, K. L., Sutton, T. M., Kinnunen, R. E. & Hoff, M. H. Overwinter survival of juvenile lake herring in relation to body size, physiological condition, energy stores, and food ration. Trans. Am. Fish. Soc. 133, 1235–1246 (2004).
Gong D. Climate of China. In Climate disasters (ed. Wang S. & Le W.) pp. 138–176 (China Meteor Press, Bei**g, 2007).
Reznick, D., Bryant, M. J. & Bashey, F. r- and k-selection revisited: the role of population regulation in life-history evolution. Ecology 83, 1509–1520 (2002).
Michaletz, P. H. Population characteristics of gizzard shad in Missouri reservoirs and their relation to reservoir productivity, mean depth, and sport fish growth. N. Am. J. Fish. Manage. 18, 114–123 (2011).
Kahn, A. T., Mautz, B. & Jennions, M. D. Females prefer to associate with males with longer intromittent organs in mosquitofish. Biol. Lett. 6, 55–58 (2010).
Heinen-Kay, J. L. & Langerhans, R. B. Predation-associated divergence of male genital morphology in a livebearing fish. J. Evol. Biol. 26, 2135–2145 (2013).
Haynes, J. L. & Cashner, R. C. Life history and population dynamics of the western mosquitofish: a comparison of natural and introduced populations. J. Fish Biol. 46, 1026–1041 (1995).
Reznick, D., Schultz, E., Morey, S. & Roff, D. On the virtue of being the first born: the influence of date of birth on fitness in the mosquitofish. Gambusia affinis. Oikos 114, 135–147 (2006).
Karlsson, J., Jonsson, A. & Jansson, M. Productivity of high-latitude lakes: climate effect inferred from altitude gradient. Global Change Biol. 11, 710–715 (2005).
Pianka, E. R. O. r- and k-selection. Am. Nat. 104, 592–597 (1970).
Bashey, F. Competition as a selective mechanism for larger offspring size in guppies. Oikos 117, 104–113 (2008).
Spoljaric, M. A. & Reimchen, T. E. 10 000 years later: Evolution of body shape in Haida Gwaii three-spined stickleback. J. Fish Biol. 70, 1484–1503 (2007).
Magnhagen, C. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–186 (1991).
Wesner, J. S., Billman, E. J., Meier, A. & Belk, M. C. Morphological convergence during pregnancy among predator and nonpredator populations of the livebearing fish Brachyrhaphis rhabdophora (Teleostei: Poeciliidae). Biol. J. Linn. Soc. 104, 386–392 (2011).
Bisazza, A., Vaccari, G. & Pilastro, A. Female mate choice in a mating system dominated by male sexual coercion. Behav. Ecol. 12, 59–64 (2001).
Cureton, J. C., Martin, R. E. & Deaton, R. Short term changes in sex ratio and density alter coercive male mating tactics. Behaviour 147, 1431–1442 (2010).
Magellan, K. & Magurran, A. E. Habitat use mediates the conflict of interest between the sexes. Anim. Behav. 72, 75–81 (2006).
Magurran, A. E. Battle of the sexes. Nature 383, 307 (1996).
Spencer, C. C. et al. Polymorphic microsatellite markers in the western mosquitofish. Gambusia affinis. Mol. Ecol. 8, 157–158 (1999).
Zane, L., Nelson, W. S., Jones, A. G. & Avise, J. C. Microsatellite assessment of multiple paternity in natural populations of a live bearing fish, Gambusia holbrooki. J. Evol. Biol. 12, 61–69 (1999).
Purcell, K. M., Lance, S. L., Jones, K. L. & Stockwell, C. A. Ten novel microsatellite markers for the western mosquitofish Gambusia affinis. Conserv. Genet. Res. 3, 361–363 (2011).
Juliano, R. O., Guerrero, R., III & Ronquillo I. The introduction of exotic aquatic species in the Philippines. In Proceedings of the workshop on introduction of exotic aquatic organisms in Asia (ed. De Silva S. S.) 83–90 (Asian Fisheries Society Spec. Publ. No 3. Asian Fisheries Society, Manila, Philippines, 1989).
Li Z. Y. & **e Y. 中 国 外 来 入 侵 种 (Alien invasive species in China) 88 (China Forestry Publishing House, Bei**g, China, 2002).
Liao I. C. & Liu H. C. Exotic aquatic species in Taiwan. In Proceedings of the workshop on introduction of exotic aquatic organisms in Asia. (ed. De Silva S. S.) 101–118 (Asian Fisheries Society Spec. Publ. No 3. Asian Fisheries Society, Manila, Philippines, 1989).
**e, Y., Li, Z., Gregg, W. P. & Li, D. Invasive species in China–an overview. Biodivers. Conserv. 10, 1317–1341 (2001).
Jourdan, J. et al. Shared and unique patterns of phenotypic diversification along a stream gradient in two congeneric species. Sci. Rep. 6, 38971 (2016).
Langerhans, R. B. & Dewitt, T. J. Shared and unique features of evolutionary diversification. Am. Nat. 164, 335–349 (2004).
Snell-Rood, E. C. & Badyaev, A. V. Ecological gradient of sexual selection: elevation and song elaboration in finches. Oecologia 157, 545–551 (2008).
Chui, C. K. S. & Doucet, S. M. A test of ecological and sexual selection hypotheses for geographical variation in coloration and morphology of golden-crowned kinglets (Regulus satrapa). J. Biogeogr. 36, 1945–1957 (2010).
Langerhans, R. B., Layman, C. A., Shokrollahi, A. M. & Dewitt, T. J. Predator-driven phenotypic diversification in Gambusia affinis. Evolution 58, 2305–2318 (2004).
Langerhans, R. B. Trade-off between steady and unsteady swimming underlies predator-driven divergence in Gambusia affinis. J. Evol. Biol. 22, 1057–1075 (2009a).
Langerhans, R. B. & Makowicz, A. M. Shared and unique features of morphological differentiation between predator regimes in Gambusia caymanensis. J. Evol. Biol. 22, 2231–2242 (2009b).
Rosen, D. E. & Gordon, M. Functional anatomy and evolution of male genitalia in poeciliid fishes. Zoologica 38, 1–47 (1953).
Langerhans, R. B. Genital evolution. In Ecology and evolution of poeciliid fishes (ed. Evans, J., Pilastro, A. & Schlupp, I.) pp. 228–240 (University of Chicago Press, Chicago, IL, 2011).
Turner, C. L. Morphogenesis of the gonopodium in. Gambusia affinis affinis J. Morphol. 69, 161–185 (1941).
Culumber, Z. W. & Tobler, M. Sex-specific evolution during the diversification of live-bearing fishes. Nat. Ecol. Evol. 1, 1185–1191 (2017).
Pincheira-Donoso, D., Hodgson, D. J., Stipala, J. & Tregenza, T. A phylogenetic analysis of sex-specific evolution of ecological morphology in Liolaemus lizards. Ecol. Res. 24, 1223–1231 (2009).
Hendry, A. P., Kelly, M. L., Kinnison, M. T. & Reznick, D. N. Parallel evolution of the sexes? Effects of predation and habitat features on the size and shape of wild guppies. J. Evol. Biol. 19, 741–754 (2006).
Riesch, R., Reznick, D. N., Plath, M. & Schlupp, I. Sex-specific local life-history adaptation in surface- and cave-dwelling Atlantic mollies (Poecilia mexicana). Sci. Rep. 6, 22968 (2016).
Fromhage, L. & Jennions, M. D. Coevolution of parental investment and sexually selected traits drives sex-role divergence. Nat. Commun. 7, 12517 (2016).
Evans, J. P. et al. Intraspecific evidence from guppies for correlated patterns of male and female genital trait diversification. Proc. Roy. Soc. Lond. Biol. Sci. 278, 2611–2620 (2011).
Head, M. L., Regina, V., Frances, J. & Jennions, M. D. Predictors of male insemination success in the mosquitofish (Gambusia holbrooki). Ecol. Evol. 5, 4999–5006 (2015).
Invernizzi, E. & Crowley, P. H. Mate density, predation risk, and the seasonal sequence of mate choices: a dynamic game. Am. Nat. 137, 567–596 (1991).
Willis, P. M., Ryan, M. J. & Rosenthal, G. G. Encounter rates with conspecific males influence female mate choice in a naturally hybridizing fish. Behav. Ecol. 22, 1234–1240 (2011).
Gilman, R. T. The evolution of sexual imprinting in socially monogamous populations. Curr. Zool. 61, 1043–1061 (2015).
Myint, O., Tsujimoto, H., Ohnishi, N., Takeyama, T. & Kohda, M. Mate availability affects female choice in a fish with paternal care: female counterstrategies against male filial cannibalism. J. Ethol. 29, 153–159 (2011).
Clark, E., Aronson, L. R. & Gordon, M. Mating behavior patterns in two sympatric species of xiphophorin fishes: their inheritance and significance in sexual isolation. Bull. Am. Mus. Nat. Hist. 103, 138–225 (1954).
Rosen, D. E. & Tucker, A. Evolution of secondary sexual characters and sexual behavior patterns in a family of viviparous fishes (Cypnnodontiformes: Poeciliidae). Copeia 1961, 201–212 (1961).
Peden, A. E. The function of gonopodial parts and behavioral pattern during copulation by Gambusia (Poeciliidae). Can. J. Zool. 50, 955–968 (2011).
Pilastro, A., Mandelli, M., Gasparini, C., Dadda, M. & Bisazza, A. Copulation duration, insemination efficiency and male attractiveness in guppies. Anim. Behav. 74, 321–328 (2007).
Constantz, G. D. Sperm competition in poeciliid fishes In Sperm Competition and the Evolution of Animal Mating Systems (ed. Smith R. l.), 465–485 (Academic Press, Orlando, FL, 1984).
Evans, J. P., Pierotti, M. & Pilastro, A. Male mating behavior and ejaculate expenditure under sperm competition risk in the eastern mosquitofish. Behav. Ecol. 14, 268–273 (2003).
Nöbel, S. & Witte, K. Public information influences sperm transfer to females in sailfin molly males. PLoS One 8, e53865 (2013).
Hosken, D. J. & Stockley, P. Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93 (2004).
Arnqvist, G. Comparative evidence for the evolution of genitalia by sexual selection. Nature 393, 784–786 (1998).
Martin, T. E. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65, 101–127 (1995).
Bassar, R. D. et al. Bridging the gap between ecology and evolution: integrating density regulation and life-history evolution. Ann. N. Y. Acad. Sci. 1206, 17–34 (2010).
Reznick, D. N. & Rodd, F. H. Life-history evolution in guppies VIII: the demographics of density regulation in guppies (Poecilia reticulata). Evolution 66, 2903–2915 (2012).
Gage, M. J. G. Risk of sperm competition directly affects ejaculate size in the Mediterranean fruit fly. Anim. Behav. 42, 1036–1037 (1991).
Oppliger, A., Hosken, D. J. & Ribi, G. Snail sperm production characteristics vary with sperm competition risk. Proc. Roy. Soc. Lond. Biol. Sci. B 265, 1527–1534 (1998).
Parker G. A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Sperm competition and sexual selection (ed. Birkhead T. R. & Møller A. P.) pp. 3–54 (Academic Press, San Diego, 1998).
Parker, G. A., Ball, M. A., Stockley, P. & Gage, M. J. G. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. Roy. Soc. Lond. Biol. Sci. 263, 1291–1297 (1996).
Leips, J., Richardson, J. M. L., Rodd, F. H. & Travis, J. Adaptive maternal adjustments of offspring size in response to conspecific density in two populations of the least killifish. Heterandria formosa. Evolution 63, 1341–1347 (2009).
Dial, T. R., Hernandez, L. P. & Brainerd, E. L. Morphological and functional maturity of the oral jaws covary with offspring size in Trinidadian guppies. Sci. Rep. 7, 5771 (2017).
Johnson, J. B. & Belk, M. C. Predation environment predicts divergent life-history phenotypes among populations of the livebearing fish Brachyrhaphis rhabdophora. Oecologia 126, 142–149 (2001).
Riesch, R., Martin, R. A. & Langerhans, R. B. Predation’s role in life-history evolution of a livebearing fish and a test of the Trexler-Deangelis model of maternal provisioning. Am. Nat. 181, 78–93 (2013).
Britton, R. H. & Moser, M. E. Size specific predation by herons and its effect on the sex-ratio of natural populations of the mosquito fish Gambusia affinis, Baird and Girard. Oecologia 53, 146–151 (1982).
Ouyang, X. et al. Characterizing a novel predator–prey relationship between native Diplonychus esakii, (Heteroptera: Belostomatidae) and invasive Gambusia affinis, (Teleostei: Poeciliidae) in central China. Int. Aquat. Res. 9, 1–11 (2017).
Menge, B. A. & Sutherland, J. P. Community regulation: variation in disturbance, competition, and predation in relation to environmental stress and recruitment. Am. Nat. 130, 730–757 (1987).
Brinsmead, J. & Fox, M. G. Morphological variation between lake- and stream-dwelling rock bass and pumpkinseed populations. J. Fish Biol. 61, 1619–1638 (2002).
Ruehl, C. B. & Dewitt, T. J. Trophic plasticity and fine-grained resource variation in populations of Western mosquitofish. Gambusia affinis. Evol. Ecol. Res. 7, 801–819 (2005).
Arendt, J. D. & Reznick, D. N. Evolution of juvenile growth rates in female guppies (Poecilia reticulata): predator regime or resource level? Proc. Roy. Soc. Lond. Biol. Sci. 272, 333–337 (2005).
Walker, J. A. Ecological morphology of lacustrine threespine stickleback Gasterosteus aculeatus L. (Gasterosteidae) body shape. Biol. J. Linn. Soc. 61, 3–50 (1997).
Trexler, J. C., Travis, J. & Mcmanus, M. Effects of habitat and body size on mortality rates of Poecilia latipinna. Ecology 73, 2224–2236 (1992).
Zulian, E., Bisazza, A. & Marin, G. Variations in male body size in natural populations of Gambusia holbrooki. Ethol. Ecol. Evol. 7, 1–10 (1995).
Bisazza, A. & Marin, G. Sexual selection and sexual size dimorphism in the eastern mosquitofish Gambusia holbrooki (Pisces: Poeciliidae). Ethol. Ecol. Evol. 2, 169–183 (1995).
Langerhans, R. B., Layman, C. A. & Dewitt, T. J. Male genital size reflects a tradeoff between attracting mates and avoiding predators in two live-bearing fish species. Proc. Natl. Acad. Sci. USA 102, 7618–7623 (2005).
Pilastro, A., Giacomello, E. & Bisazza, A. Sexual selection for small size in male mosquitofish (Gambusia holbrooki). Proc. Roy. Soc. Lond. Biol. Sci. 264, 1125–1129 (1997).
Weissman, D. B., Judge, K. A., Williams, S. C., Whitman, D. W. & Lee, V. F. Small-male mating advantage in a species of Jerusalem cricket (Orthoptera: Stenopelmatinae: Stenopelmatus). J. Orthopt. Res. 17, 321–332 (2008).
Mclachlan, A. J. & Allen, D. F. Male mating success in Diptera: advantages of small size. Oikos 48, 11–14 (1987).
Moore, M. P., Riesch, R. & Martin, R. A. The predictability and magnitude of life-history divergence to ecological agents of selection: a meta-analysis in livebearing fishes. Ecol. Lett. 19, 435–442 (2016).
Gomes, J. L. Jr. & Monteiro, L. R. Size and fecundity variation in populations of Poecilia vivipara Block & Schneider (Teleostei; Poeciliidae) inhabiting an environmental gradient. J. Fish Biol. 71, 1799–1809 (2007).
Jennions, M. & Telford, S. Life-history phenotypes in populations of Brachyrhaphis episcopi (Poeciliidae) with different predator communities. Oecologia 132, 44–50 (2002).
Gorini-Pacheco, B., Zandonà, E. & Mazzoni, R. Predation effects on matrotrophy, superfetation and other life history traits in. Phalloceros harpagos. Ecol. Freshw. Fish 2017, 1–11 (2017).
Maclaren, R. D. & Daniska, D. Female preferences for dorsal fin and body size in **phophorus helleri: further investigation of the LPA bias in poeciliid fishes. Behaviour 145, 897–913 (2008).
Plath, M., Schlupp, I., Parzefall, J. & Riesch, R. Female choice for large body size in the cave molly, Poecilia mexicana (Poeciliidae, Teleostei): influence of species- and sex-specific cues. Behaviour 144, 1147–1160 (2007).
Gabor, C. R. & Page, R. Female preference for large males in sailfin mollies, Poecilia latipinna: the importance of predation pressure and reproductive status. Acta Ethol. 6, 7–12 (2003).
Tobler, M., Schlupp, I. & Plath, M. Does divergence in female mate choice affect male size distributions in two cave fish populations? Biol. Lett. 4, 452–454 (2008).
Bisazza, A. & Marin, G. Male size and female mate choice in the eastern mosquitofish (Gambusia holbrooki: Poeciliidae). Copeia 3, 730–735 (1991).
Stearns, S. C. A new view of life-history evolution. Oikos 35, 266–281 (1980).
Lodge, D. M. Biological invasions—lessons for ecology. Trends Ecol. Evol. 8, 133–137 (1993).
Levine, J. M. Species diversity and biological invasions: relating local process to community pattern. Science 288, 852–854 (2000).
Ehrenfeld, J. G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 41, 59–80 (2010).
Clavero, M., Brotons, L., Pons, P. & Sol, D. Prominent role of invasive species in avian biodiversity loss. Biol. Conserv. 142, 2043–2049 (2009).
Kimbro, D. L. et al. Invasive species cause large-scale loss of native California oyster habitat by disrupting trophic cascades. Oecologia 160, 563–575 (2009).
Hermoso, V., Clavero, M., Blanco-Garrido, F. & Prenda, J. Invasive species and habitat degradation in Iberian streams: an analysis of their role in freshwater fish diversity loss. Ecol. Appl. 21, 175–188 (2011).
Reznick, D. N. & Ghalambor, C. K. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113, 183–198 (2001).
Arnett, H. A. Sources of ecologically important trait variation in Mosquitofish (Gambusia affinis and Gambusia holbrooki). PhD thesis, University of Maine, Maine, USA, 190 pp (2016).
Tan Y. J. & Tong, H. Y. The status of the exotic aquatic organisms in China. In: Proceedings of the workshop on introduction of exotic aquatic organisms in Asia (ed. De Silva S. S.) pp. 35–43 (Asian Fisheries Society Spec. Publ. No 3. Asian Fisheries Society, Manila, Philippines, 1989).
Krumholz, L. A. Reproduction in the western mosquitofish, Gambusia affinis affinis (Baird & Girard), and its use in mosquito control. Ecol. Monogr. 18, 1–43 (1948).
Rees, B. E. Attributes of the mosquito fish in relation to mosquito control. Proc. Calif. Mosq. Contr. Assoc. 26, 71–75 (1958).
Jann, P., Blanckenhorn, W. U. & Ward, P. I. Temporal and microspatial variation in the intensities of natural and sexual selection in the yellow dung fly. Scathophaga stercoraria. J. Evol. Biol. 13, 927–938 (2000).
Svensson, E. I., Eroukhmanoff, F. & Friberg, M. Effects of natural and sexual selection on adaptive population divergence and premating isolation in a damselfly. Evolution 60, 1242–1253 (2006).
Ghalambor, C. K. et al. Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature 525, 372–375 (2015).
Ghalambor, C. K., Mckay, J. K., Carroll, S. P. & Reznick, D. N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 (2007).
Ancel, L. W. Undermining the Baldwin expediting effect: does phenotypic plasticity accelerate evolution? Theor. Popul. Biol. 58, 307–319 (2000).
Castro, G., Myers, J. P. & Ricklefs, R. E. Ecology and energetics of sandlerlings migrating to four latitudes. Ecology 73, 833–844 (1992).
Conover, D. O. & Present, T. M. C. Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia 83, 316–324 (1990).
Perez, K. O. & Munch, S. B. Extreme selection on size in the early lives of fish. Evolution 64, 2450–2457 (2010).
Fraser, D. F. & Gilliam, J. F. Feeding under predation hazard: response of the guppy and Hart’s rivulus from sites with contrasting predation hazard. Behav. Ecol. Sociobiol. 21, 203–209 (1987).
Lin, J. & Zhang, Q. Characteristics of temperature and precipitation climate state change in the south and the north of China and its influence of climate monitoring. Progressus Inquisitiones de Mutatione Climatis 11, 281–287 (2015).
Fu, C., Wu, J., Chen, J., Wu, Q. & Lei, G. Freshwater fish biodiversity in the Yangtze river basin of China: patterns, threats and conservation. Biodivers. Conserv. 12, 1649–1685 (2003).
Hu, B., Wang, H., Yang, Z. & Sun, X. Temporal and spatial variations of sediment rating curves in the Changjiang (Yangtze river) basin and their implications. Quatern. Int. 230, 34–43 (2011).
Excoffier, L. & Lischer, H. E. L. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Res. 10, 564–567 (2010).
Goudet, J. FSTAT version 2.9.3.2, a program to estimate and test gene diversities and fixation indices. Institute of Ecology, Lausanne, Switzerland (2002).
Van Ousterhout, C., Hutchinson, W. F., Wills, D. & Shipley, P. MICRO-CHECKER: software for identifying and correcting genoty** errors in microsatellite data. Mol. Ecol. Notes 4, 535–538 (2004).
Chapuis, M. & Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 24, 621–631 (2007).
Piry, S., Luikart, G. & Cornuet, J. M. BOTTLENECK: A computer program for detecting recent reductions in the effective population size using allele frequency data. J. Hered. 90, 502–503 (1999).
Luikart, G. & Cornuet, J. M. Empirical evaluation of a test for identifying recently bottlenecked populations from allele frequency data. Conserv. Biol. 12, 228–237 (1998).
Pritchard, J. K., Wen, X. & Falush, D. Documentation for structure software: Version 2.3 (2009).
Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 14, 2611–2620 (2005).
Nei, M. & Chesser, R. K. Estimation of fixation indices and gene diversities. Ann. Hum. Genet. 47, 253–259 (1983).
Page, R. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12, 357–358 (1996).
Peakall, R. & Smouse, P. E. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288–295 (2006).
Peakall, R. & Smouse, P. E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformat. 28, 2537–2539 (2012).
Riesch, R., Schlupp, I., Langerhans, R. B. & Plath, M. Shared and unique patterns of embryo development in extremophile poeciliids. PLoS One 6, e27377 (2011).
Rohlf, F. J. tpsUtil, version 1.70. Department of Ecology and Evolution, State University of New York at Stony Brook. Online at http://life.bio.sunysb.edu/morph/ (2016a).
Rohlf, F. J. tpsDig, version 2.26. Department of Ecology and Evolution, State University of New York at Stony Brook. Online at http://life.bio.sunysb.edu/morph/ (2016b).
Klingenberg, C. P. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Res. 11, 353–357 (2011).
Acknowledgements
We are indebted to Y. Zhong and H. Cao for collecting specimens in Beihai and Hangzhou. E. M. Wörner kindly shared dissection protocols. Financial support came from Northwest A&F University (Z111021403) and from the Province of Shaanxi (A289021611, both to M. Plath).
Author information
Authors and Affiliations
Contributions
X.O. and M.P. designed the study. X.O., J.G., M.X., B.L. and L.Z. collected the data. X.O., J.G. M.X. and M.P. performed data analyses. X.O., J.G., M.P., J.J. and R.R. wrote the manuscript. All authors contributed to manuscript writing and approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ouyang, X., Gao, J., **e, M. et al. Natural and sexual selection drive multivariate phenotypic divergence along climatic gradients in an invasive fish. Sci Rep 8, 11164 (2018). https://doi.org/10.1038/s41598-018-29254-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29254-4
- Springer Nature Limited
This article is cited by
-
Genetic and phenotypic diversification in a widespread fish, the Sailfin Molly (Poecilia latipinna)
BMC Ecology and Evolution (2024)
-
Species distribution modeling combined with environmental DNA analysis to explore distribution of invasive alien mosquitofish (Gambusia affinis) in China
Environmental Science and Pollution Research (2024)
-
Climatic and geographic variation as a driver of phenotypic divergence in reproductive characters and body sizes of invasive Gambusia holbrooki
Aquatic Sciences (2022)
-
Small-scale phenotypic differentiation along complex stream gradients in a non-native amphipod
Frontiers in Zoology (2019)