Abstract
Large-scale single-cell ‘omics profiling is being used to define a complete catalogue of brain cell types, something that traditional methods struggle with due to the diversity and complexity of the brain. But this poses a problem: How do we organise such a catalogue - providing a standard way to refer to the cell types discovered, linking their classification and properties to supporting data? Cell ontologies provide a partial solution to these problems, but no existing ontology schemas support the definition of cell types by direct reference to supporting data, classification of cell types using classifications derived directly from data, or links from cell types to marker sets along with confidence scores. Here we describe a generally applicable schema that solves these problems and its application in a semi-automated pipeline to build a data-linked extension to the Cell Ontology representing cell types in the Primary Motor Cortex of humans, mice and marmosets. The methods and resulting ontology are designed to be scalable and applicable to similar whole-brain atlases currently in preparation.
Similar content being viewed by others
Introduction
The large-scale application of omics profiling techniques at the single-cell level is producing enormous volumes of data. Cell ontologies are poised to play a critical role in making these data searchable and integratable1. At the same time, the application of these profiling techniques is revolutionising our understanding of cell types and cellular heterogeneity2,3. The impact of this revolution is especially dramatic for the brain. Due to the complex cellular architecture of the brain, traditional qualitative, categorical methods of classifying neurons based on location, morphology, marker expression and function have not achieved a coherent, unified view of granular brain cell types and their classifications. This has begun to change with the application of massively parallel single-cell or nucleus RNA sequencing (sc/snRNAseq) methods to the brain, combined with multimodal transcriptomic techniques such as Patch-seq.4. The BRAIN Initiative Cell Census Network (BICCN) recently completed a comprehensive, multimodal cell census and atlas of the primary motor cortex across multiple species5,6,7. This takes the approach of treating consensus clustering of similar cells from single nucleus RNA-seq data from multiple experiments as a ground truth for defining cell types and their classification. The resulting cell type hierarchies serve as anchors for alignment of data from other modalities, allowing spatial localization, morphology, electrical properties, chromatin accessibility, and other features of cell types to be recorded and compared across species. Evidence from systems in which a more comprehensive classification of cell types has been achieved by classical methods than has been possible in the brain suggests that the classifications resulting from sc/snRNAseq analysis align closely with classically defined types8.
This poses challenges for standard approaches to ontology development. How are we to integrate cell types defined with reference to clusters of transcriptomically similar cells into cell ontologies in which cell type/classes are defined using simple, categorical assertions about their morphological and functional properties, location and marker expression? How can we do this in a way that is transparent about the origins and evidence for these classifications? How can we enable ontology users to leverage the data used to define and classify reference cell types in the ontology to classify cell types represented in their own data?
Here we describe a solution to these challenges in the form of a template-driven ontology generation pipeline and an ontology of cell types defined in the BICCN mini-atlas, Brain Data Standards Ontology (BDSO), that forms part of the Provisional Cell Ontology3, which extends the Cell Ontology9 with potential new cell types from single cell analysis. Ontologies should serve as both an easily searchable source of terms for annotation and a data structure supporting organisation, search and navigation of annotated data. We demonstrate the utility of our ontology for this via its application to the organisation, search and navigation of data about cells in the mini-atlas on the Allen Cell Type Knowledge Explorer web app.
Results
Brain data standards ontology design
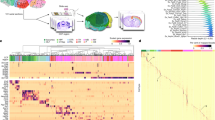
One of the outputs of the BICCN mini-atlas10 is a standardized representation of cell clusters (CCN) and the hierarchical relationships between them that constitute the ground-truth for cell-types defined in the atlas. The clusters and their hierarchical arrangement derive from unsupervised, hierarchical clusterings of single-cell transcriptomic and epigenetic profiles of the primary motor cortex in mouse, human, and marmoset10,11. Each individual hierarchical clustering (referred to here as a taxonomy) is either created from a single data set (e.g., in marmoset) or through a consensus of two (human) or many (mouse) data sets. Using mouse transcriptomics clusterings as an anchor, morphological and electrophysiological profiles of single-cells are mapped to omics-based types using Patch-seq data7. Finally, comparison of clusters across species is used to generate cross-species map**s and grou**s of clusters which represent putative homology grou**s10,11. All of this information is available in a standard format (common cell type nomenclature taxonomy files, here referred to as CCN taxonomy files) developed by the BICCN to represent mammalian brain cell type taxonomies and the relationships between them12.
To produce a set of definitional characteristics of the cell types identified in these taxonomies, a minimum set of markers that can be used to distinguish cells in that cluster from those in other clusters in the same taxonomy was produced using the NS-Forest algorithm13. Taking the clusters as ground truth for all cell types present in the primary motor cortex, the combined expression of each marker set should be necessary and sufficient to identify the corresponding cell type in the context of the primary motor cortex.
The BDSO is built as a faithful representation of the BICCN mini-atlas cell type taxonomies (Fig. 1). In order to achieve this, we first devised a schema to represent taxonomies in Web Ontology Language, OWL214, the formal language we use for constructing ontologies. OWL2 makes a distinction between individuals, e.g., an individual neuron depicted in a micrograph, and classes, e.g., the class of all Chandelier neurons. Each taxonomy is represented in BDSO as a collection of OWL Individuals, with each Individual representing a cluster of single-cell transcriptomes and retaining all original metadata in the CCN taxonomy file from which it is derived. Hierarchical clustering is represented by relating these individuals to each other via a transitive subcluster_of relation.
Each taxonomy has many more nodes than it would be reasonable to create classes for. In order to select useful intermediate nodes for representation, taxonomy authors of the BICCN mini-atlas flagged nodes to generate a 3-level hierarchy with the most granular level consisting of all leaf nodes10. We generated cell classes for all tagged clusters, apart from some high-level grou**s (e.g. all cells, non-neuronal, etc.) that would not make sense as a cell type term as they are overly generalised. Each of these classes is linked formally to a cluster individual using a standard pattern in OWL that can be used by standard OWL reasoning software to automatically build a classification hierarchy for the BDSO classes (see Fig. 2 and the next section for more details). Lastly, we treated cross-species map**s between cell types as putative homology map**s, by using the relation in_historical_homology_relationship_with15 (imported from the OBO relations ontology) in a pairwise manner.
Graph illustrating the BDSO schema. This graph shows the relationship of the BDSO classes (Brain Data Standards Ontology nodes, light blue circles) to OWL Individuals (Taxonomy nodes, brown circles) representing clusters in the data-driven taxonomy used as input and to the build process, to classes in the Cell Ontology (green circles) and from external ontologies (imported terms box) representing species (NCBITaxon), brain region (UBERON), morphology (PATO), and markers (Ensembl/PRO). NS-Forest marker combinations are represented through sets, with individual markers being part_of them. The right side of the figure shows links to potentially homologous cell type classes (Cross-species box) using the relation (OWL objectProperty) ‘in historical homology relationship with’ and cross-region terms (Cross-region box).
To integrate the BDSO with existing ontologies, classes defined for intermediate nodes in the hierarchy are further classified using classes in CL, which we have extended as required (e.g., see ‘L5 extratelencephalic’ class in Fig. 2). These include classes that are defined by expression of classical marker genes (e.g., VIP-expressing GABAergic neurons), morphology (pyramidal) or projection pattern (extratelencephalic projecting), mapped based on co-collected transcriptomic profiles10. The BDSO also reuses existing ontologies to represent species (NCBITaxon16), brain region (UBERON17), morphology (PATO), and marker genes (Ensembl/PRO18,19). All relationships added use OBO standard relations from the OBO relations ontology and follow or extend standard schemas used by CL (Fig. 2). In addition to tightly integrating these terms with CL, this approach maximises the potential for making data annotated with BDSO interoperable with the many other datasets annotated with these ontologies.
Designing an automated pipeline
Manually building an ontology to represent the huge amount of data from the BICCN mini-atlas is impractical, error-prone, and unscalable. It was therefore imperative to harness automated tools to build the BDSO. To build the BDSO, we use CCN taxonomy files, NS-Forest marker gene map**s and reference gene lists as input to a semi-automated pipeline. The pipeline takes advantage of the schema described in Fig. 3 to build a hierarchy that mirrors the cluster hierarchy (see L5 ET in Figs. 1 & 3 for example implementation). The BDSO is built using the Ontology Development Kit20 and uses standard ontology term templating systems21,22 to generate labels, definitions and synonyms for BDSO terms and to add CL classifications and relationships (more strictly, existential restrictions in Web Ontology Language (OWL)) recording location (using Uberon terms17), species (using NCBI taxonomy terms16), markers, projection patterns and morphologies (see Fig. 4 for examples). The results of NS-Forest analysis, ingested via standardised TSV files, are automatically consumed by the pipeline and integrated into the ontology (see section below). Manual curation such as map** to CL terms, adding cell properties (morphology, projections, etc.) were kept to a minimum and done via templates to ensure consistency and scalability.
Representative schema for data-driven classification. Blue nodes (i1–3) are OWL individuals representing clusters of single-cell transcriptomes, while tan nodes (c1, c2) are OWL classes representing cell types. Hierarchical clustering is represented using the transitive subcluster_of relation (objectProperty) to link individuals. Each class is defined by reference to a cluster individual (i), via the relation (objectProperty) as equivalent to (any) cell that has_examplar (value) i. Reasoning via a chain of these two properties (bottom and right sides of the diagram above) is sufficient to infer that c3 has_examplar value i1 and so, combined with the assertion that it is a (type of) cell, fulfils the conditions required to be a subclass of i1.
Example of an automatically generated class displayed in the Protege ontology browser. In this example, we show L5 Extratelencephalic (ET), which is a grou** class. The label, definition, and set of synonyms are auto-generated from OWL templates using a Dead Simple OWL Design Patterns (DOSDP) system. Automatic axiomatisation includes brain region, species, NS-Forest markers, projection pattern, morphology, named markers, and has_exemplar_data link to taxonomy node (cluster), using a reification pattern. This results in the reasoner classifying this class under L5 extratelencephalic projecting glutamatergic cortical neuron (based on automated axiomatisation of brain region and projection pattern), and primary motor cortex pyramidal cell (based on automated axiomatisation of morphology and brain region). has_characterzing_marker_set schema for NS-Forest is also shown.
Representing data and analysis results
The BDSO uses the direct results of data analyses as evidence for the existence of cell type classes. To reflect this, and to allow users direct access to the data that justifies the categorical assertions that we make, we link the ontology clusters to datasets (expression matrices) available on Nemo (https://assets.nemoarchive.org/dat-ch1nqb7), and we include the quantitative data that support categorical assertions made in the ontology, where this data is available. Currently, we include a measure of the accuracy of classification using NS-Forest marker F-Beta scores and we plan to incorporate measures of transcriptomic similarity to support homology assertions. CCN taxonomy files include a measure of confidence in the division into (sibling) subclusters, plotted as height in dendrogram views. We retain this measure, along with all other metadata, attached to individual clusters.
Each set of NS-Forest markers should theoretically be necessary and sufficient for identifying a cell type with high precision within the dataset used to define them. In the case of the mini-atlas, the datasets correspond to all cells with a soma located in the primary motor cortex of some specified species and so should be necessary and sufficient for identifying the cell type within that anatomical context more generally. We also have evidence that they are useful for detecting the same cell type in other brain regions: In many cases, the markers identified by NS-Forest in the primary motor cortex, are expressed in equivalent cell types found in another cortical brain region (middle temporal gyrus)23 however the NS-Forest algorithm typically finds other sets of makers in these cases.
We record this context as a restriction on the class using a has_soma_location to the brain region and represent NS-Forest markers through an NS-Forest set class, ‘S’ in the example below, with marker genes as parts (See Figs. 1 and 3):
{C} has_characterizing_marker_set some {S}; {S} has_part some gene 1; {S} has_part some gene 2
This approach allows us to record multiple marker sets for each cell type, which may be essential in future, given the many competing methods available for defining cell type markers. The intermediate node allows for clear grou** of marker sets in knowledge graphs (see Fig. 2). We also use the node to record Fβ scores for each set - recording the accuracy of classification using the markers on the reference transcriptomic datasets. We do this through a custom annotation property ‘fbeta_confidence_score’ that is annotated on the marker set class.
We rejected an alternative approach of using an EquivalentClass axiom with clauses to restrict for location and NS-Forest markers to formally specify necessary and sufficient conditions. Equivalent class axioms are used to drive automated classification of subclasses and individuals using reasoning. BDSO terms already have one EquivalentClass axiom, defining classes with reference to data and used to convert data driven classification in the taxonomy into OWL classification. The addition of equivalentClass axioms defining cell types by NS forest markers + classifications could potentially cause additional unwanted classifications. Even with precision of classification of individual cells with these markers at 98%, a rare cell type, comprising less than 2% of cells, might be misclassified. This solution would also not be compatible with adding additional, alternative marker sets based on other algorithms.
Ontology content summary
The latest release (2022-04-27 Release) of the BDSO component (which PCL imports) contains 913 individuals, out of which 890 are taxonomy nodes (individuals also include datasets), and 112447 classes (including genes and NS-Forest sets), out of which 1384 have the PCL namespace and 555 are cell types. The remaining terms are imported from OBO ontologies into PCL. All object properties used are imported from RO as per OBO foundry guidelines.
Application
A key function of the BDSO is to support organisation, navigation and searching of data in a community-accessible view of the cell types defined in the BICCN mini-atlas of the mammalian primary motor cortex10 through a web-based application (web-app) that integrates cell type descriptions and related data, known as the “Cell Type Knowledge Explorer” (Fig. 5). Each page in this web-app corresponds to a cell type defined with reference to a cluster in one of the BICCN taxonomies represented in the BDSO, and features a wide range of data and analysis from multiple cross integrated datasets. The aim of the ontology-driven search and navigation tools is to support access to these pages in the web-app.
Screenshots of the alpha version of the Cell Type Knowledge Explorer web app, incorporating search and navigation functionality driven by the BDSO. (a) An overview of the web app with the ontology incorporated into it. Red arrows show zoomed in version and directional links. (b) An example of autocomplete search, which also allows search by synonyms. (c) Information about the cell type incorporates ontology identifiers, ontology symbols, and ontology names. (d) A list of synonyms generated by ontology annotations and extra curated synonyms. (e) A list of NS-Forest markers with links out to their identifiers.org pages. (f) Semantic tags of the cell type corresponding to species, brain region, and cell properties such as morphology (pyramidal) and projection pattern (extratelencephalic). Clicking on one of these panels drives faceted search through the search bar seen in (g).
While expressiveness of ontology formats such as OWL is an advantage for semantic data processing, OWL is complicated to develop applications with and has limited tooling. Graph databases like neo4j, and indexed document stores such as SOLR and ElasticSearch, provide a more tractable, fast way to drive web applications. For this purpose, we extended a library, neo4j2owl24, developed for the Virtual Fly Brain project25,26, that ensures logical projection of OWL ontologies into labelled property graphs. Neo4j2owl imports OWL ontologies into Neo4j in a way that preserves entailments and annotations, but not the syntactic complexities of OWL. It also supports the addition of semantic tags, in the form of simple strings attached to classes and individuals, driven by OWL DL or SPARQL queries. We use this semantic tag system to provide an application-specific, gross classification that provides additional information about classes in a useful form to users and can be used to drive faceted search. For example, we can tag all classes corresponding to subclasses of GABAergic neuron, or all classes fulfilling an OWL DL query for classes of neuron with pyramidal morphology (see Fig. 5f). The full Knowledge Graph can be accessed at http://purl.obolibrary.org/obo/pcl/bds/kg/, and can be accessed without a username or password (leaving the fields blank and clicking connect).
An illustration of the resulting property graph is shown in Fig. 2. These property graphs allow applications such as the Cell Type Knowledge Explorer to use the ontology data to populate parts of the application and enable full-text and faceted search functions.
Ontology-based navigation and search functions are provided through two mechanisms - autocomplete (which takes advantage of curation of synonyms in the ontology) and faceted search (Fig. 5). Autocomplete allows users to search for cell-type ontology terms, displaying a list of lexical matches for users to choose from (Fig. 5b). Faceted search of Cell Type Knowledge Explorer works via a set of tags corresponding to gross classifications (e.g. GABAergic), intrinsic properties (e.g. pyramidal morphology) and extrinsic properties (brain region location, species) of cell types, added to cell type neo4j nodes via OWL DL queries of the underlying ontologies. Currently, implementation of this works through automatically adding the term to the search bar and allowing the free-text search to complete the search (Fig. 5f,g). However, this approach is unlikely to scale as the content of Cell Type Knowledge Explorer grows. There are plans to allow users to take better advantage of faceted browsing using semantic tags via a results page that can be refined via combinations of semantic tags combined with lexical search, allowing users to find neurons by any combination of location, morphology, species, neurotransmitter and name/synonym substring.
Discussion
The BDSO is a faithful representation of the data-driven, consensus cell type classification that includes the BICCN mini-atlas of the mammalian motor cortex10. By using a schema that defines classes logically via links to an OWL representation of data and analyses, we can use OWL to directly leverage the data-driven taxonomy of the miniatlas to classify cell types in BDSO using OWL reasoning. As a result, classes retain direct links to the data and analyses that define them and the origins of this classification are transparent and insulated from the manual editing process that might alter or obfuscate them. Using templated specification of ontology classes, the BDSO build process is scalable and extensible and allows a flexible mix of automation and manual curation. It also makes it possible to update as new, improved versions of data-driven classifications of the same cell types are released. The linked data can potentially be used to replicate analyses and to map cell types defined in BDSO to other datasets (e.g., using Azimuth27, FR-match23). The addition of NS-Forest markers13, representing minimal markers for distinguishing, with high confidence, cell types from other cell types defined in the analysis, provides a simple mechanism for map** cell types from third-party transcriptomics data to the BDSO.
In future, we plan to incorporate measures of transcriptomic similarity in support of homology assertions and a measure of confidence for data-driven taxonomy nodes. We will also incorporate contextual information about the nature of these measures. While the absolute values of these measures are inevitably specific to the datasets/analysis they come from, they are at least usable for intra-dataset comparisons. As a broader consensus and whole-brain datasets emerge, we expect NS-Forest F-Beta scores and taxonomy node confidence measures to be informative of which cell types we consider stable and replicable.
While the approach described meets many of the requirements for a scalable approach to cell type representation, some challenges remain. The current representation lacks links to transcriptomic data from Patch-seq data used to map morphologically defined types. Using transcriptomic clustering as ground truth for an ontology also comes with its inherent challenges. Penetrance of marker expression and location to a specific cortical layer varies across clusters, so all/some quantified assertions of marker expression in OWL will always be an approximation and will always require either automated or qualitative assessment of thresholds. Finally, nomenclature issues frequently arise when data-driven classifications are mapped onto classically-defined classes. For example, the literature is full of references to VIP-expressing GABAergic neurons, identified using VIP as a marker, but clustering defines a broader group of related GABAergic neurons including some subtypes that do not express VIP, at least not at levels detectable by snRNAseq in the adult mouse.
The transcriptomic approach potentially allows the definition of transcriptomically defined, species-neutral grou** classes. We decided against adding these because the resulting classifications are not likely to remain stable as more species are added to the analysis, although this may change in future with large-scale analyses using many species. It is also likely to be challenging to map these classes to the more traditionally defined species-neutral cell type ontology classes.
Another challenge comes from working with nomenclature defined by researchers. Terminology that makes sense in the limited local context of a dataset can be confusing to users viewing it in the broader, integrated context of an ontology. In the primary motor cortex mini-atlas datasets used for this work, names given to cell types in human and marmoset were derived from the names of the mouse cell types, even where that name implies properties (e.g., marker expressions) that do not apply. For example, the Sncg cluster in marmoset is aligned to that of mouse Scng cluster but contains many cell types that do not necessarily express Sncg (Fig. 6). To make this clear we rename these terms following the pattern mouse {x} like, e.g., (Mouse Sncg)-like (Marmoset).
Example of a cell type name that is derived from the names of the mouse cell types. The Marmoset (Callithrix jacchus) cell type taxonomy is aligned to the mouse cell type taxonomy, resulting in a “sncg grou**” that contains cell types that do not necessarily express Sncg. To make this clear, the class was renamed (Mouse Sncg)-like.
Lastly, as efforts to expand scRNAseq cell ty** to the entire brain, there is a crucial need for upstream standardisation and validation in order to efficiently scale up what we have presented in this paper. Tooling that allows biologists to annotate cell types with existing terms created through the BDSO, automated checks for quality control, and consensus on data formats, nomenclatures, and version control are all required if we are to effectively manage the huge input of data that is inevitable from such work.
The general schema/approach that we describe for defining and classifying cell types with reference to exemplar data is both scalable and broadly applicable across data sources and types. It could, for example, be applied to the definition and classification of Drosophila neuron types by morphology and location which has become standard in Drosophila neurobiology28,29. The ontology build pipeline described here has so far been applied to one additional dataset (snRNAseq of the medial temporal gyrus30) and will soon be applied to a taxonomy for the whole mouse brain. While the pipeline is tailored to using taxonomies that follow the CCN standard12 as input, the modular nature of its design means it could easily be adapted to any other hierarchical representation of cell type/classification linked to data.
Ultimately, our proposal should be evaluated on the basis of its usefulness of ontology product outputs in cell type annotation and projection, and in driving atlasing products such as the Allen Cell Type Knowledge Explorer. By this criteria, it has already succeeded. However, wider reach will require time and outreach to the community.
Conclusion
We have defined a generally applicable schema for defining and classifying cell types using reference data and linking to markers and confidence scores derived from that data. The BDSO acts as a functional tool for managing data from the BICCN mini atlas project, underlying the search and navigation of the Cell Type Knowledge Explorer web application, and provides a controlled vocabulary for future annotations. Beyond its practical function, it is also an example of how ontologies can harness automation to process the large volumes of analyses that are inevitable with the rise of sc/snRNAseq methods. Crucially, the work on the BDSO has highlighted the need for good tooling and integration into the early steps of the processes of sc/snRNAseq experiments.
The BDSO is a practical first step to generating ontologies from taxonomies representing sc/snRNAseq-based cell ty** in the brain, one that is not only important for the tools it underlies (e.g. Cell Type Knowledge Explorer), but crucially needed for annotation of the increasing amount of sc/snRNAseq datasets coming from the brain. As we head towards full brain coverage of cell ty** by sc/scRNAseq, BDSO presents a good template that can be further extended with clearer provenance, more direct links to data, and better representation of confidence; extensions that will require close collaborations with data producers.
Methods
Data source
Input to the ontology was derived from data from the BICCN mini-atlas10 and scRNAseq of the human middle temporal gyrus30. NS-Forest analysis was done as previously described13 using gene lists available from either NCBI gene16 or Ensembl18.
Development strategy
BDSO is developed based on the OBO Foundry31,32 and FAIR33 principles. Ontology terms were reused as much as possible (see results section) with all relationships used coming from the relations ontology and design patterns following or extending those used in the Cell Ontology. The BDSO is fully compliant with OBO Foundry standards and has been included as an ontology in the OBO Foundry.
Templating systems
The templating systems used in the automated pipeline are ROBOT21 (used to generate individuals) and DOSDP22. Briefly, information is extracted from the CCN taxonomy files and translated into template files that are processed either through ROBOT templates to generate individuals, or template files for classes where a curator manually curates additional information (e.g. map**s to CL cell types, morphology, etc.) which is then processed, together with NS-Forest markers, using DOSDP. These files are then merged as part of the pipeline for the final product.
Provisional cell ontology
We updated the Provisional Cell Ontology to follow OBO Foundry standards by using a pipeline based on the ontology development kit20. Earlier, manually generated releases of PCL shared terms with the version described here, but used non-standard IDs and schema. In order to support map** of data previously annotated with PCL and references to PCL terms in previous publications3,11,30, we mapped all original IDs to current OBO standard persistent URLs, using OBO standard map**s for obsoleted terms.
Endpoints
As well as being available for downloading from a persistent URL (http://purl.obolibrary.org/obo/pcl.owl) and available for browsing on widely used ontology platforms including the Ontology Lookup service and Ontobee, the BDSO can be searched and queried via a REST API (http://purl.obolibrary.org/obo/pcl/bds/api/). These endpoints encapsulate the representational complexities of the underlying knowledge and property graphs and serve the ontology in web-friendly formats such as JSON. Using these endpoints, users can search for ontology terms, access their details and navigate through the ontology using relationships between concepts. Solr is used at the backend to provide enhanced full-text search and reduced service response times. The created Solr indexes are published publicly (https://github.com/obophenotype/brain_data_standards_queries).
BDSO analysis
Statistics of metadata of BDSO were done using SPARQL queries with ROBOT21 on the BDSO component. SPARQL queries used can be found in the repository (https://github.com/obophenotype/brain_data_standards_ontologies/tree/master/src/sparql).
Figures generation
Figure 1 ontology visualisation was generated by using the Ontology Access Kit34 and dendrogram section was provided by the BICCN10. Figures 4 & 6 uses screenshots from Protege35. Figure 5 uses screenshots from the Cell Type Knowledge Explorer web app (https://knowledge.brain-map.org/celltypes/).
Data availability
All data used in the BDSO is publicly available and can be found in the original papers as well as NeMO archive links available in the ontology. Code and source data used to generate the ontology is publicly available at GitHub (https://github.com/obophenotype/brain_data_standards_ontologies and https://github.com/JCVenterInstitute/NSForest). Primary motor cortex taxonomies are also publicly available (https://github.com/AllenInstitute/MOp_taxonomies_ontology).
Code availability
The BDSO is generated using a dedicated ontology build pipeline, built as an extension to the Ontology Development Kit20, but released as a component of the PCL, with all terms having PCL IDs. Previous releases of PCL3,11,30 represented some of the same cell types as the current release but used a different, less formal schema and a different ID system3,11,30. We have obsoleted these terms and provided a map**, within PCL, to replacement terms allowing continued support for previous work annotated using PCL terms.
The BDSO’s code base is available at GitHub (https://github.com/obophenotype/brain_data_standards_ontologies) including documentation of the full technology stack and details of the approach. The latest release of the ontology is available for download from http://purl.obolibrary.org/obo/pcl/bds/bds.owl and is hosted on the EMBL-EBI ontology lookup service (OLS)36 at https://www.ebi.ac.uk/ols/ontologies/pcl. OLS provides ontology search, browsing, visualisation capabilities and enables web services driven programmatic access to the BDSO.
Change history
28 April 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41597-023-02165-4
References
Osumi-Sutherland, D. et al. Cell type ontologies of the Human Cell Atlas. Nat. Cell Biol. 23, 1129–1135 (2021).
Nguyen, Q. H., Pervolarakis, N., Nee, K. & Kessenbrock, K. Experimental Considerations for Single-Cell RNA Sequencing Approaches. Front Cell Dev Biol 6, 108 (2018).
Bakken, T. et al. Cell type discovery and representation in the era of high-content single cell phenoty**. BMC Bioinformatics 18, 559 (2017).
Cadwell, C. R. et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat. Biotechnol. 34, 199–203 (2016).
Gouwens, N. W. et al. Integrated Morphoelectric and Transcriptomic Classification of Cortical GABAergic Cells. Cell 183 (2020).
Berg, J. et al. Human neocortical expansion involves glutamatergic neuron diversification. Nature 598 (2021).
Scala, F. et al. Phenotypic variation of transcriptomic cell types in mouse motor cortex. Nature https://doi.org/10.1038/s41586-020-2907-3 (2020).
Shekhar, K. et al. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 166, 1308–1323.e30 (2016).
Diehl, A. D. et al. The Cell Ontology 2016: enhanced content, modularization, and ontology interoperability. J. Biomed. Semantics 7, 44 (2016).
BRAIN Initiative Cell Census Network (BICCN). A multimodal cell census and atlas of the mammalian primary motor cortex. Nature 598, 86–102 (2021).
Bakken, T. E. et al. Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature 598, 111–119 (2021).
Miller, J. A. et al. Common cell type nomenclature for the mammalian brain. Elife 9 (2020).
Aevermann, B. D. et al. A machine learning method for the discovery of minimum marker gene combinations for cell-type identification from single-cell RNA sequencing. Genome Res., https://doi.org/10.1101/gr.275569.121 (2021).
Hitzler, P. et al. OWL 2 web ontology language primer. W3C recommendation 27, 123 (2009).
Mabee, P. M. et al. A Logical Model of Homology for Comparative Biology. Syst. Biol. 69, 345–362 (2020).
Sayers, E. W. et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 48, D9–D16 (2020).
Mungall, C. J., Torniai, C., Gkoutos, G. V., Lewis, S. E. & Haendel, M. A. Uberon, an integrative multi-species anatomy ontology. Genome Biol. 13, R5 (2012).
Howe, K. L. et al. Ensembl 2021. Nucleic Acids Res. 49, D884–D891 (2021).
Natale, D. A. et al. The Protein Ontology: a structured representation of protein forms and complexes. Nucleic Acids Res. 39, D539–45 (2011).
Matentzoglu, N. et al. Ontology Development Kit: a toolkit for building, maintaining and standardizing biomedical ontologies. Database 2022, baac087 (2022).
Jackson, R. C. et al. ROBOT: A Tool for Automating Ontology Workflows. BMC Bioinformatics 20, 407 (2019).
Osumi-Sutherland, D., Courtot, M., Balhoff, J. P. & Mungall, C. Dead simple OWL design patterns. J. Biomed. Semantics 8, 18 (2017).
Zhang, Y., Aevermann, B., Gala, R. & Scheuermann, R. H. Cell type matching in single-cell RNA-sequencing data using FR-Match. Sci. Rep. 12, 9996 (2022).
Matentzoglu, N., Kir, H., Osumi-Sutherland, D. & Court, R. VirtualFlyBrain/neo4j2owl: 1.1.24-PRE. https://doi.org/10.5281/zenodo.7082530 (2022).
Milyaev, N. et al. The Virtual Fly Brain browser and query interface. Bioinformatics 28, 411–415 (2012).
Osumi-Sutherland, D., Costa, M., Court, R. & O’Kane, C. Virtual Fly Brain-Using OWL to support the map** and genetic dissection of the Drosophila brain. in Proceedings of OWLED 2014 (ed. C. M. Keet) 85–96 (2014).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Bates, A. S., Janssens, J., Jefferis, G. S. & Aerts, S. Neuronal cell types in the fly: single-cell anatomy meets single-cell genomics. Curr. Opin. Neurobiol. 56, 125–134 (2019).
Costa, M., Manton, J. D., Ostrovsky, A. D., Prohaska, S. & Jefferis, G. S. X. E. NBLAST: Rapid, Sensitive Comparison of Neuronal Structure and Construction of Neuron Family Databases. Neuron 91, 293–311 (2016).
Hodge, R. D. et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019).
Jackson, R. et al. OBO Foundry in 2021: operationalizing open data principles to evaluate ontologies. Database 2021 (2021).
Smith, B. et al. The OBO Foundry: coordinated evolution of ontologies to support biomedical data integration. Nat. Biotechnol. 25, 1251–1255 (2007).
Wilkinson, M. D. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 3, 160018 (2016).
Mungall, C. et al. INCATools/ontology-access-kit: v0.1.22. https://doi.org/10.5281/zenodo.6643629 (2022).
Musen, M. A., Protégé Team. The Protégé Project: A Look Back and a Look Forward. AI Matters 1, 4–12 (2015).
Jupp, S., Burdett, T., Leroy, C. & Parkinson, H. E. A new Ontology Lookup Service at EMBL-EBI. SWAT4LS 2, 118–119 (2015).
Acknowledgements
This work was funded by NIMH:1RF1MH123220-01 - “A Community Framework for Data-driven Brain Transcriptomic Cell Type Definition, Ontology, and Nomenclature.” We thank Maryann Martone and Carol Thompson for their invaluable contributions to discussions of the work described here. This work was funded by NIMH/NIH:1U24MH114827-01 - “A Community Resource for Single Cell Data in the Brain.”
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The ontology and knowledge graph described in this paper were constructed by S.Z.K.T. & H.K. under supervision by D.O.S., using pipelines developed by H.K., N.M. & D.O.S. and using inputs generated by J.A.M. (taxonomies) and B.D.A., Y.Z., & R.H.S. (NS-Forest analysis). Cell Type Knowledge Explorer was developed by J.A.M., E.S.L., M.J.H., T.S.M., P.L.R., R.E.A.S., B.S., J.V., & A.Y. using APIs developed by H.K. & D.O.S. Semantic schema design was a joint effort between S.Z.K.T., H.K. and D.O.S. with important contributions from R.H.S., B.D.A., J.A.M., P.L.R., C.J.M. and T.G. and was guided by discussion with all other authors. Work on the Provisional Cell Ontology extends work from R.H.S. & B.D.A. with updated pipelines developed by S.Z.K.T., H.K., N.M., & D.O.S. This manuscript was largely written by S.Z.K.T., D.O.S., H.K., R.H.S. & J.A.M. with edits and suggestions from other authors.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing financial interests or potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, S.Z.K., Kir, H., Aevermann, B.D. et al. Brain Data Standards - A method for building data-driven cell-type ontologies. Sci Data 10, 50 (2023). https://doi.org/10.1038/s41597-022-01886-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-022-01886-2
- Springer Nature Limited