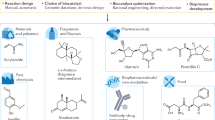
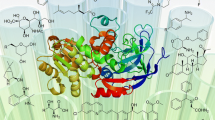
Abstract
Living systems contain a vast network of metabolic reactions, providing a wealth of enzymes and cells as potential biocatalysts for chemical processes. The properties of protein and cell biocatalysts—high selectivity, the ability to control reaction sequence and operation in environmentally benign conditions—offer approaches to produce molecules at high efficiency while lowering the cost and environmental impact of industrial chemistry. Furthermore, biocatalysis offers the opportunity to generate chemical structures and functions that may be inaccessible to chemical synthesis. Here we consider developments in enzymes, biosynthetic pathways and cellular engineering that enable their use in catalysis for new chemistry and beyond.




Similar content being viewed by others
References
Hafner, J., MohammadiPeyhani, H., Sveshnikova, A., Scheidegger, A. & Hatzimanikatis, V. Updated ATLAS of biochemistry with new metabolites and improved enzyme prediction power. ACS Synth. Biol. 9, 1479–1482 (2020).
Schmid, A. et al. Industrial biocatalysis today and tomorrow. Nature 409, 258–268 (2001).
Koeller, K. M. & Wong, C.-H. Enzymes for chemical synthesis. Nature 409, 232–240 (2001).
Hönig, M., Sondermann, P., Turner, N. J. & Carreira, E. M. Enantioselective chemo- and biocatalysis: partners in retrosynthesis. Angew. Chem. Int. Ed. 56, 8942–8973 (2017).
Romero, E. et al. Enzymatic late-stage modifications: better late than never. Angew. Chem. Int. Ed. 60, 16824–16855 (2021).
Wu, S., Snajdrova, R., Moore, J. C., Baldenius, K. & Bornscheuer, U. T. Biocatalysis: enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. 60, 88–119 (2021).
Savile, C. K. et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 329, 305–309 (2010). This work was a landmark in the field, highlighting the utility of transaminases for the preparation of chiral amines and its impact in reducing cost and waste generation at the commercial production scale of sitagliptin at Merck.
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Bergman, R. G. C–H activation. Nature 446, 391–393 (2007).
Dalton, T., Faber, T. & Glorius, F. C–H activation: toward sustainability and applications. ACS Cent. Sci. 7, 245–261 (2021).
Chang, M. C. Y. & Keasling, J. D. Production of isoprenoid pharmaceuticals by engineered microbes. Nat. Chem. Biol. 2, 674–681 (2006).
Stout, C. N. & Renata, H. Reinvigorating the chiral pool: chemoenzymatic approaches to complex peptides and terpenoids. Acc. Chem. Res. 54, 1143–1156 (2021).
Sono, M., Roach, M. P., Coulter, E. D. & Dawson, J. H. Heme-containing oxygenases. Chem. Rev. 96, 2841–2888 (1996).
Guengerich, F. P. Mechanisms of cytochrome P450-catalyzed oxidations. ACS Catal. 8, 10964–10976 (2018).
Jung, S. T., Lauchli, R. & Arnold, F. H. Cytochrome P450: taming a wild type enzyme. Curr. Opin. Biotechnol. 22, 809–817 (2011).
Park, J. et al. Fungal cytochrome P450 database. BMC Genomics 9, 402 (2008).
Wang, H. et al. PCPD: plant cytochrome P450 database and web-based tools for structural construction and ligand docking. Synth. Syst. Biotechnol. 6, 102–109 (2021).
Narayan, A. R. H. et al. Enzymatic hydroxylation of an unactivated methylene C–H bond guided by molecular dynamics simulations. Nat. Chem. 7, 653–660 (2015).
Gally, C., Nestl, B. M. & Hauer, B. Engineering Rieske non-heme iron oxygenases for the asymmetric dihydroxylation of alkenes. Angew. Chem. Int. Ed. 54, 12952–12956 (2015).
Zhang, K., El Damaty, S. & Fasan, R. P450 fingerprinting method for rapid discovery of terpene hydroxylating P450 catalysts with diversified regioselectivity. J. Am. Chem. Soc. 133, 3242–3245 (2011).
Li, F., Deng, H. & Renata, H. Remote B-ring oxidation of sclareol with an engineered P450 facilitates divergent access to complex terpenoids. J. Am. Chem. Soc. 144, 7616–7621 (2022).
Paddon, C. J. & Keasling, J. D. Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 12, 355–367 (2014).
Agarwal, V. et al. Enzymatic halogenation and dehalogenation reactions: pervasive and mechanistically diverse. Chem. Rev. 117, 5619–5674 (2017).
Latham, J., Brandenburger, E., Shepherd, S. A., Menon, B. R. K. & Micklefield, J. Development of halogenase enzymes for use in synthesis. Chem. Rev. 118, 232–269 (2018).
Glenn, W. S., Nims, E. & O’Connor, S. E. Reengineering a tryptophan halogenase to preferentially chlorinate a direct alkaloid precursor. J. Am. Chem. Soc. 133, 19346–19349 (2011).
Andorfer, M. C., Park, H. J., Vergara-Coll, J. & Lewis, J. C. Directed evolution of RebH for catalyst-controlled halogenation of indole C–H bonds. Chem. Sci. 7, 3720–3729 (2016).
Blasiak, L. C., Vaillancourt, F. H., Walsh, C. T. & Drennan, C. L. Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis. Nature 440, 368–371 (2006).
Matthews, M. L. et al. Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase. Nat. Chem. Biol. 10, 209–215 (2014).
Neugebauer, M. E. et al. A family of radical halogenases for the engineering of amino-acid-based products. Nat. Chem. Biol. https://doi.org/10.1038/s41589-019-0355-x (2019).
Gomez, C. A., Mondal, D., Du, Q., Chan, N. & Lewis, J. C. Directed evolution of an iron(II)- and α-ketoglutarate-dependent dioxygenase for site-selective azidation of unactivated aliphatic C−H bonds. Angew. Chem. Int. Ed. 135, e202301370 (2023).
Neugebauer, M. E. et al. Reaction pathway engineering converts a radical hydroxylase into a halogenase. Nat. Chem. Biol. 18, 171–179 (2022).
Mitchell, A. J. et al. Structure-guided reprogramming of a hydroxylase to halogenate its small molecule substrate. Biochemistry 56, 441–444 (2017).
Coelho, P. S., Brustad, E. M., Kannan, A. & Arnold, F. H. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science 339, 307–310 (2013). This work provided a paradigm for engineering enzymatic reaction chemistry for abiotic synthetic transformations, using insight into organic chemistry to develop diazo regents for delivering elements for P450 group transfer.
Dydio, P., Key, H. M., Hayashi, H., Clark, D. S. & Hartwig, J. F. Chemoselective, enzymatic C–H bond amination catalyzed by a cytochrome P450 containing an Ir(Me)-PIX cofactor. J. Am. Chem. Soc. 139, 1750–1753 (2017).
Huang, J. et al. Complete integration of carbene-transfer chemistry into biosynthesis. Nature 617, 403–408 (2023). This work shows how the discovery of biosynthetic pathways can facilitate the complete biological reproduction of synthetic enzyme chemistry. In this case, the biosynthesis of azaserine and the parallel expression of an engineered P450 enables carbene-transfer chemistry inside of living cells.
Kan, S. B. J., Huang, X., Gumulya, Y., Chen, K. & Arnold, F. H. Genetically programmed chiral organoborane synthesis. Nature 552, 132–136 (2017).
Kan, S. B. J., Lewis, R. D., Chen, K. & Arnold, F. H. Directed evolution of cytochrome c for carbon–silicon bond formation: bringing silicon to life. Science 354, 1048–1051 (2016).
Farwell, C. C., McIntosh, J. A., Hyster, T. K., Wang, Z. J. & Arnold, F. H. Enantioselective imidation of sulfides via enzyme-catalyzed intermolecular nitrogen-atom transfer. J. Am. Chem. Soc. 136, 8766–8771 (2014).
Zhou, Q., Chin, M., Fu, Y., Liu, P. & Yang, Y. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450. Science 374, 1612–1616 (2021).
Dydio, P. et al. An artificial metalloenzyme with the kinetics of native enzymes. Science 354, 102–106 (2016).
Sreenilayam, G., Moore, E. J., Steck, V. & Fasan, R. Metal substitution modulates the reactivity and extends the reaction scope of myoglobin carbene transfer catalysts. Adv. Synth. Catal. 359, 2076–2089 (2017).
Zhang, J., Huang, X., Zhang, R. K. & Arnold, F. H. Enantiodivergent α-amino C–H fluoroalkylation catalyzed by engineered cytochrome P450s. J. Am. Chem. Soc. 141, 9798–9802 (2019).
Nam, D. et al. Enantioselective synthesis of α-trifluoromethyl amines via biocatalytic N–H bond insertion with acceptor–acceptor carbene donors. J. Am. Chem. Soc. 144, 2590–2602 (2022).
Goldberg, N. W., Knight, A. M., Zhang, R. K. & Arnold, F. H. Nitrene transfer catalyzed by a non-heme iron enzyme and enhanced by non-native small-molecule ligands. J. Am. Chem. Soc. 141, 19585–19588 (2019).
Zhao, Q. et al. Engineering non-haem iron enzymes for enantioselective C(sp3)–F bond formation via radical fluorine transfer. Nat. Synth. https://doi.org/10.1038/s44160-024-00507-7 (2024).
Zetzsche, L. E. & Narayan, A. R. H. Broadening the scope of biocatalytic C–C bond formation. Nat. Rev. Chem. 4, 334–346 (2020).
Takayama, S., McGarvey, G. J. & Wong, C.-H. Microbial aldolases and transketolases: new biocatalytic approaches to simple and complex sugars. Annu. Rev. Microbiol. 51, 285–310 (1997).
Schrittwieser, J. H., Velikogne, S., Hall, M. & Kroutil, W. Artificial biocatalytic linear cascades for preparation of organic molecules. Chem. Rev. 118, 270–348 (2018).
Fang, J., Hait, D., Head-Gordon, M. & Chang, M. C. Y. Chemoenzymatic platform for synthesis of chiral organofluorines based on type II aldolases. Angew. Chem. Int. Ed. 58, 11841–11845 (2019).
Fang, J., Turner, L. E. & Chang, M. C. Y. Biocatalytic asymmetric construction of secondary and tertiary fluorides from β-fluoro-α-ketoacids. Angew. Chem. Int. Ed. 61, e202201602 (2022).
Dunn, B. J. & Khosla, C. Engineering the acyltransferase substrate specificity of assembly line polyketide synthases. J. R. Soc. Interface 10, 20130297 (2013).
Koryakina, I. et al. Poly specific trans-acyltransferase machinery revealed via engineered acyl-CoA synthetases. ACS Chem. Biol. 8, 200–208 (2013).
Sirirungruang, S. et al. Engineering site-selective incorporation of fluorine into polyketides. Nat. Chem. Biol. 18, 886–893 (2022).
Rittner, A. et al. Chemoenzymatic synthesis of fluorinated polyketides. Nat. Chem. 14, 1000–1006 (2022).
Mazzaferro, L. S., Hüttel, W., Fries, A. & Müller, M. Cytochrome P450-catalyzed regio- and stereoselective phenol coupling of fungal natural products. J. Am. Chem. Soc. 137, 12289–12295 (2015).
Zetzsche, L. E. et al. Biocatalytic oxidative cross-coupling reactions for biaryl bond formation. Nature 603, 79–85 (2022).
Schultz, E. E., Braffman, N. R., Luescher, M. U., Hager, H. H. & Balskus, E. P. Biocatalytic Friedel–Crafts alkylation using a promiscuous biosynthetic enzyme. Angew. Chem. Int. Ed. 58, 3151–3155 (2019).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Mayer, C., Gillingham, D. G., Ward, T. R. & Hilvert, D. An artificial metalloenzyme for olefin metathesis. Chem. Commun. 47, 12068–12070 (2011).
Abe, S. et al. Control of the coordination structure of organometallic palladium complexes in an apo-ferritin cage. J. Am. Chem. Soc. 130, 10512–10514 (2008).
Filice, M. et al. Preparation of an immobilized lipase-palladium artificial metalloenzyme as catalyst in the Heck reaction: role of the solid phase. Adv. Synth. Catal. 357, 2687–2696 (2015).
Jeschek, M. et al. Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 537, 661–665 (2016). This work shows that artificial metalloenzymes engineered using a tethered synthetic ruthenium catalyst for olefin metathesis can be expressed and evolved in living cells, providing a potential roadmap for evolution of activities using completely abiotic cofactors.
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016). This work was a landmark for discovering new modes of enzymatic catalysis, using photocatalysis as a creative approach to generate reactive radical intermediates to carry out interesting and diverse synthetic transformations in an enzyme active site.
Cheng, L. et al. Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis. Science 381, 444–451 (2023).
Anbarasan, P. et al. Integration of chemical catalysis with extractive fermentation to produce fuels. Nature 491, 235–239 (2012). This work showed how rethinking mature fermentation processes using chemical ingenuity allows for the scalable production of chemicals. In this case, the products of the acetone–butanol–ethanol fermentation could be combined with Guerbet chemistry to produce mid-chain-length hydrocarbons as biofuels.
Wang, Z. Q. et al. A dual cellular–heterogeneous catalyst strategy for the production of olefins from glucose. Nat. Chem. 13, 1178–1185 (2021).
Pyser, J. B. et al. Stereodivergent, chemoenzymatic synthesis of azaphilone natural products. J. Am. Chem. Soc. 141, 18551–18559 (2019).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Ivanenkov, Y. A., Zagribelnyy, B. A. & Aladinskiy, V. A. Are we opening the door to a new era of medicinal chemistry or being collapsed to a chemical singularity? J. Med. Chem. 62, 10026–10043 (2019).
Fink, T. & Reymond, J.-L. Virtual exploration of the chemical universe up to 11 atoms of C, N, O, F: assembly of 26.4 million structures (110.9 million stereoisomers) and analysis for new ring systems, stereochemistry, physicochemical properties, compound classes, and drug discovery. J. Chem. Inf. Model. 47, 342–353 (2007).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Davison, E. K. & Brimble, M. A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin. Chem. Biol. 52, 1–8 (2019).
Stone, S., Newman, D. J., Colletti, S. L. & Tan, D. S. Cheminformatic analysis of natural product-based drugs and chemical probes. Nat. Prod. Rep. 39, 20–32 (2022).
Chen, Y., Garcia de Lomana, M., Friedrich, N.-O. & Kirchmair, J. Characterization of the chemical space of known and readily obtainable natural products. J. Chem. Inf. Model. 58, 1518–1532 (2018).
Lawson, A. D. G., MacCoss, M. & Heer, J. P. Importance of rigidity in designing small molecule drugs to tackle protein–protein interactions (PPIs) through stabilization of desired conformers. J. Med. Chem. 61, 4283–4289 (2018).
Over, B. et al. Natural-product-derived fragments for fragment-based ligand discovery. Nat. Chem. 5, 21–28 (2013).
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M. & Supuran, C. T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216 (2021).
Barelier, S. et al. Increasing chemical space coverage by combining empirical and computational fragment screens. ACS Chem. Biol. 9, 1528–1535 (2014).
Ertl, P., Altmann, E. & McKenna, J. M. The most common functional groups in bioactive molecules and how their popularity has evolved over time. J. Med. Chem. 63, 8408–8418 (2020).
Hert, J., Irwin, J. J., Laggner, C., Keiser, M. J. & Shoichet, B. K. Quantifying biogenic bias in screening libraries. Nat. Chem. Biol. 5, 479–483 (2009).
Tang, M.-C., Zou, Y., Watanabe, K., Walsh, C. T. & Tang, Y. Oxidative cyclization in natural product biosynthesis. Chem. Rev. 117, 5226–5333 (2017).
Grigalunas, M. et al. Natural product fragment combination to performance-diverse pseudo-natural products. Nat. Commun. 12, 1883 (2021).
Grigalunas, M., Brakmann, S. & Waldmann, H. Chemical evolution of natural product structure. J. Am. Chem. Soc. 144, 3314–3329 (2022).
Kim, S. S. & Sattely, E. S. Dirigent proteins guide asymmetric heterocoupling for the synthesis of complex natural product analogues. J. Am. Chem. Soc. 143, 5011–5021 (2021).
Lloyd, C. T. et al. Discovery, structure and mechanism of a tetraether lipid synthase. Nature 609, 197–203 (2022).This work demonstrates an interesting mechanism by which two radicals can be generated and coupled using radical SAM enzymes to achieve sp3–sp3 coupling.
Cacho, R. A., Chooi, Y.-H., Zhou, H. & Tang, Y. Complexity generation in fungal polyketide biosynthesis: a spirocycle-forming P450 in the concise pathway to the antifungal drug griseofulvin. ACS Chem. Biol. 8, 2322–2330 (2013).
Sib, A. & Gulder, T. A. M. Stereoselective total synthesis of bisorbicillinoid natural products by enzymatic oxidative dearomatization/dimerization. Angew. Chem. Int. Ed. 56, 12888–12891 (2017).
Baker Dockrey, S. A., Lukowski, A. L., Becker, M. R. & Narayan, A. R. H. Biocatalytic site- and enantioselective oxidative dearomatization of phenols. Nat. Chem. 10, 119–125 (2018). This work showcases a creative chemical approach to using flavin adenine dinucleotide (FAD)-dependent monooxygenases for oxidative dearomatization as a strategy for creating stereocentres.
Yoshikuni, Y., Ferrin, T. E. & Keasling, J. D. Designed divergent evolution of enzyme function. Nature 440, 1078–1082 (2006). This work shows how the plasticity of terpene synthase active sites can be utilized to engineer the production of a wide range of different sesquiterpenes.
Johnson, L. A., Dunbabin, A., Benton, J. C. R., Mart, R. J. & Allemann, R. K. Modular chemoenzymatic synthesis of terpenes and their analogues. Angew. Chem. 132, 8564–8568 (2020).
Crawford, J. M. et al. Structural basis for biosynthetic programming of fungal aromatic polyketide cyclization. Nature 461, 1139–1143 (2009).
Montalbán-López, M. et al. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 38, 130–239 (2021).
Maresh, J. J. et al. Strictosidine synthase: mechanism of a Pictet−Spengler catalyzing enzyme. J. Am. Chem. Soc. 130, 710–723 (2008).
Chen, M., Liu, C.-T. & Tang, Y. Discovery and biocatalytic application of a PLP-dependent amino acid γ-substitution enzyme that catalyzes C–C bond formation. J. Am. Chem. Soc. 142, 10506–10515 (2020).
Sutherland, E., Harding, C. J. & Czekster, C. M. Active site remodelling of a cyclodipeptide synthase redefines substrate scope. Commun. Chem. 5, 101 (2022).
Jeon, B., Wang, S.-A., Ruszczycky, M. W. & Liu, H. Natural [4 + 2]-cyclases. Chem. Rev. 117, 5367–5388 (2017).
Jamieson, C. S., Ohashi, M., Liu, F., Tang, Y. & Houk, K. N. The expanding world of biosynthetic pericyclases: cooperation of experiment and theory for discovery. Nat. Prod. Rep. 36, 698–713 (2019).
Gao, L. et al. Enzymatic control of endo- and exo-stereoselective Diels–Alder reactions with broad substrate scope. Nat. Catal. 4, 1059–1069 (2021).
Ohashi, M. et al. An enzymatic Alder-ene reaction. Nature 586, 64–69 (2020).
Liu, Z. et al. An NmrA-like enzyme-catalysed redox-mediated Diels–Alder cycloaddition with anti-selectivity. Nat. Chem. 15, 526–534 (2023).
Andrews, P. R., Smith, G. D. & Young, I. G. Transition-state stabilization and enzymic catalysis. Kinetic and molecular orbital studies of the rearrangement of chorismate to prephenate. Biochemistry 12, 3492–3498 (1973).
Zhang, X. et al. Divergent synthesis of complex diterpenes through a hybrid oxidative approach. Science 369, 799–806 (2020). This work reports a tour de force in combining chemical synthesis with enzymatic late-stage functionalization to provide complex molecules with structural diversity.
Doyon, T. J. et al. Chemoenzymatic o-quinone methide formation. J. Am. Chem. Soc. 141, 20269–20277 (2019).
Wlodek, A. et al. Diversity oriented biosynthesis via accelerated evolution of modular gene clusters. Nat. Commun. 8, 1206 (2017).
Cimermancic, P. et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 158, 412–421 (2014).
van Santen, J. A. et al. The natural products atlas: an open access knowledge base for microbial natural products discovery. ACS Cent. Sci. 5, 1824–1833 (2019).
Medema, M. H., de Rond, T. & Moore, B. S. Mining genomes to illuminate the specialized chemistry of life. Nat. Rev. Genet. https://doi.org/10.1038/s41576-021-00363-7 (2021).
Blin, K. et al. antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res. 49, W29–W35 (2021).
Kim, L. J. et al. Prospecting for natural products by genome mining and microcrystal electron diffraction. Nat. Chem. Biol. 17, 872–877 (2021).
Lee, J., Simurdiak, M. & Zhao, H. Reconstitution and characterization of aminopyrrolnitrin oxygenase, a Rieske N-oxygenase that catalyzes unusual arylamine oxidation. J. Biol. Chem. 280, 36719–36728 (2005).
Nelp, M. T. & Bandarian, V. A single enzyme transforms a carboxylic acid into a nitrile through an amide intermediate. Angew. Chem. Int. Ed. 54, 10627–10629 (2015).
Woodyer, R. D. et al. Heterologous production of fosfomycin and identification of the minimal biosynthetic gene cluster. Chem. Biol. 13, 1171–1182 (2006).
Sugai, Y., Katsuyama, Y. & Ohnishi, Y. A nitrous acid biosynthetic pathway for diazo group formation in bacteria. Nat. Chem. Biol. 12, 73–75 (2016).
O’Hagan, D. & Deng, H. Enzymatic fluorination and biotechnological developments of the fluorinase. Chem. Rev. 115, 634–649 (2015).
Galván, A. E. et al. Identification of the biosynthetic gene cluster for the organoarsenical antibiotic arsinothricin. Microbiol. Spectr. 9, e00502–e00521 (2021).
Kayrouz, C. M., Huang, J., Hauser, N. & Seyedsayamdost, M. R. Biosynthesis of selenium-containing small molecules in diverse microorganisms. Nature 610, 199–204 (2022).
Zhu, X., Liu, J. & Zhang, W. De novo biosynthesis of terminal alkyne-labeled natural products. Nat. Chem. Biol. 11, 115–120 (2015).
Marchand, J. A. et al. Discovery of a pathway for terminal-alkyne amino acid biosynthesis. Nature 567, 420–424 (2019).
Walker, M. C. et al. Expanding the fluorine chemistry of living systems using engineered polyketide synthase pathways. Science 341, 1089–1094 (2013).
Du, Y.-L., He, H.-Y., Higgins, M. A. & Ryan, K. S. A heme-dependent enzyme forms the nitrogen–nitrogen bond in piperazate. Nat. Chem. Biol. 13, 836–838 (2017).
Ng, T. L., Rohac, R., Mitchell, A. J., Boal, A. K. & Balskus, E. P. An N-nitrosating metalloenzyme constructs the pharmacophore of streptozotocin. Nature 566, 94–99 (2019).
Del Rio Flores, A. et al. Biosynthesis of triacsin featuring an N-hydroxytriazene pharmacophore. Nat. Chem. Biol. 17, 1305–1313 (2021).
Winkler, C. K., Schrittwieser, J. H. & Kroutil, W. Power of biocatalysis for organic synthesis. ACS Cent. Sci. 7, 55–71 (2021).
McKinnie, S. M. K. et al. Total enzyme syntheses of napyradiomycins A1 and B1. J. Am. Chem. Soc. 140, 17840–17845 (2018).
Li, J., Li, F., King-Smith, E. & Renata, H. Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids. Nat. Chem. 12, 173–179 (2020).
Li, W., McArthur, J. B. & Chen, X. Strategies for chemoenzymatic synthesis of carbohydrates. Carbohydr. Res. 472, 86–97 (2019).
DeAngelis, P. L., Liu, J. & Linhardt, R. J. Chemoenzymatic synthesis of glycosaminoglycans: re-creating, re-modeling and re-designing nature’s longest or most complex carbohydrate chains. Glycobiology 23, 764–777 (2013).
Palluk, S. et al. De novo DNA synthesis using polymerase-nucleotide conjugates. Nat. Biotechnol. 36, 645–650 (2018).
Lee, H. H., Kalhor, R., Goela, N., Bolot, J. & Church, G. M. Terminator-free template-independent enzymatic DNA synthesis for digital information storage. Nat. Commun. 10, 2383 (2019).
Korman, T. P., Opgenorth, P. H. & Bowie, J. U. A synthetic biochemistry platform for cell free production of monoterpenes from glucose. Nat. Commun. 8, 15526 (2017).
Sherkhanov, S. et al. Isobutanol production freed from biological limits using synthetic biochemistry. Nat. Commun. 11, 4292 (2020). This work is one of a series of papers demonstrating that entire metabolic pathways can be reconstituted from glucose in vitro, enabling the total biosynthesis of a broad range of molecules from biomass using isolated enzyme, resulting in yields higher than tolerated by living systems.
Valliere, M. A., Korman, T. P., Arbing, M. A. & Bowie, J. U. A bio-inspired cell-free system for cannabinoid production from inexpensive inputs. Nat. Chem. Biol. 16, 1427–1433 (2020).
Schwander, T., Schada von Borzyskowski, L., Burgener, S., Cortina, N. S. & Erb, T. J. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 354, 900–904 (2016).
Huffman, M. A. et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 366, 1255–1259 (2019). This work is a landmark in demonstrating the use of nine enzymes in an abiotic cascade to produce the drug islatravir by scientists at Merck, highlighting a creative pathway designed to produce a nucleotide analogue that contains both a fluorine and an alkyne substituent. Notably this cascade is less than half the number of steps of preceding chemical routes and provides improved stereochemical purity and atom economy at every step.
Finnigan, W., Hepworth, L. J., Flitsch, S. L. & Turner, N. J. RetroBioCat as a computer-aided synthesis planning tool for biocatalytic reactions and cascades. Nat. Catal. 4, 98–104 (2021).
Schomburg, I. et al. The BRENDA enzyme information system–from a database to an expert system. J. Biotechnol. 261, 194–206 (2017).
Sun, D. et al. EnzyMine: a comprehensive database for enzyme function annotation with enzymatic reaction chemical feature. Database https://doi.org/10.1093/database/baaa065 (2020).
Burgener, S., Luo, S., McLean, R., Miller, T. E. & Erb, T. J. A roadmap towards integrated catalytic systems of the future. Nat. Catal. 3, 186–192 (2020).
Kiefer, A. F., Liu, Y., Gummerer, R., Jäger, C. & Deska, J. An artificial in vitro metabolism to angiopterlactone B inspired by traditional retrosynthesis. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202301178 (2023).
D’Agostino, P. M., Seel, C. J., Ji, X., Gulder, T. & Gulder, T. A. M. Biosynthesis of cyanobacterin, a paradigm for furanolide core structure assembly. Nat. Chem. Biol. 18, 652–658 (2022).
Stephanopoulos, G. N., Aristidou, A. A. & Nielsen, J. Metabolic Engineering: Principles and Methodologies (Academic Press, 1998).
Keasling, J. D. Synthetic biology for synthetic chemistry. ACS Chem. Biol. 3, 64–76 (2008).
Liao, J. C., Mi, L., Pontrelli, S. & Luo, S. Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 14, 288–304 (2016).
Lee, S. Y. et al. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2, 18–33 (2019).
**ong, M., Schneiderman, D. K., Bates, F. S., Hillmyer, M. A. & Zhang, K. Scalable production of mechanically tunable block polymers from sugar. Proc. Natl Acad. Sci. USA 111, 8357–8362 (2014).
Srinivasan, P. & Smolke, C. D. Biosynthesis of medicinal tropane alkaloids in yeast. Nature 585, 614–619 (2020).
Olson, D. G., McBride, J. E., Joe Shaw, A. & Lynd, L. R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 23, 396–405 (2012).
Liew, F. E. et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat. Biotechnol. 40, 335–344 (2022).
Yoshida, S. et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 351, 1196–1199 (2016).
Lu, H. et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 604, 662–667 (2022). This work demonstrates the highly successful evolution of an enzyme that can depolymerize and recycle a plastic. By using an enzyme to hydrolyze polyethylene terephthalate (PET), monomers are cleanly produced that can be repolymerized.
Seifrid, M. et al. Autonomous chemical experiments: challenges and perspectives on establishing a self-driving lab. Acc. Chem. Res. 55, 2454–2466 (2022).
Buchberger, A. R., DeLaney, K., Johnson, J. & Li, L. Mass spectrometry imaging: a review of emerging advancements and future insights. Anal. Chem. 90, 240–265 (2018).
Markin, C. J. et al. Revealing enzyme functional architecture via high-throughput microfluidic enzyme kinetics. Science 373, eabf8761 (2021).
Holland-Moritz, D. A. et al. Mass activated droplet sorting (MADS) enables high-throughput screening of enzymatic reactions at nanoliter scale. Angew. Chem. Int. Ed. 59, 4470–4477 (2020). This work describes the development of a high-throughput liquid-droplet screening system that can analyse 25-nl samples at a rate of 15,000 in 6 h by mass spectrometery, greatly increasing the potential for a general screening approach for engineering, characterization and discovery of enzymes.
Li, C. et al. Single-cell multi-omics in the medicinal plant Catharanthus roseus. Nat. Chem. Biol. 19, 1031–1041 (2023). This work highlights how technological advances in analytical chemistry can be creatively applied to biological processes to reveal information. Here the authors combine transcriptomics and metabolomics with single-cell resolution in a medicinal plant to rapidly identify and discover entire biosynthetic pathways while tracking the movement of intermediates between compartments.
Kim, L. J. et al. Prospecting for natural products by genome mining and microcrystal electron diffraction. Nat. Chem. Biol. 17, 872–877 (2021).This work demonstrates a pipeline for the discovery of natural products, allowing for the detection of the products of ‘silent’ biosynthetic gene clusters while rapidly determining their structure via microcrystal electron diffraction.
Gross, L. et al. Atomic force microscopy for molecular structure elucidation. Angew. Chem. Int. Ed. 57, 3888–3908 (2018).
Garg, N. K., Caspi, D. D. & Stoltz, B. M. The total synthesis of (+)-dragmacidin F. J. Am. Chem. Soc. 126, 9552–9553 (2004).
Lichman, B. R. et al. The evolutionary origins of the cat attractant nepetalactone in catnip. Sci. Adv. 6, eaba0721 (2020).
Acknowledgements
We thank the National Institutes of Health (R01 GM134271) and the National Science Foundation (CLP 2204014) for their generous support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and editing of this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Nicholas Turner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kissman, E.N., Sosa, M.B., Millar, D.C. et al. Expanding chemistry through in vitro and in vivo biocatalysis. Nature 631, 37–48 (2024). https://doi.org/10.1038/s41586-024-07506-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07506-w
- Springer Nature Limited