Abstract
The abnormal accumulation of α-synuclein (α-syn) is a crucial factor for the onset and pathogenesis of Parkinson’s disease (PD), and the autophagy-lysosome pathway (ALP) contributes to α-syn turnover. AMP-activated protein kinase (AMPK) and the mammalian target of rapamycin (mTOR) regulate autophagy by initiating the macroautophagy cascade and promoting lysosomal biogenesis via increased transcription factor EB (TFEB) activity. Hence, activation of AMPK-mTOR-TFEB axis-mediated autophagy might promote α-syn clearance in PD. Harmol is a β-carboline alkaloid that has been extensively studied in a variety of diseases but rarely in PD models. In this study, we aimed to evaluate the effect and underlying mechanism of harmol in PD models in vitro and in vivo. We show that harmol reduces α-syn via ALP in a dose- and time-dependent manner in cell model that overexpressed human A53T mutant α-syn. We also demonstrate that harmol promotes the translocation of TFEB into the nucleus and accompanies the restoration of autophagic flux and lysosomal biogenesis. Importantly, harmol improves motor impairment and down-regulates α-syn levels in the substantia nigra and prefrontal cortex in the α-syn transgenic mice model. Further studies revealed that harmol might activate ALP through AMPK-mTOR-TFEB to promote α-syn clearance. These in vitro and in vivo improvements demonstrate that harmol activates the AMPK-mTOR-TFEB mediated ALP pathway, resulting in reduced α-syn, and suggesting the potential benefit of harmol in the treatment of PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, clinically characterized by motor abnormalities (rest tremor, rigidity, bradykinesia, shuffling gait, and postural instability) and non-motor symptoms (depression, anxiety, dementia, sleep disorder)1. This characteristic movement restriction reduces the ability of PD patients to work, and lessens their quality of life. Indeed, late-stage PD patients often cannot take care of themselves, which significantly burdens individual caretakers, families, and society2. The loss of dopaminergic neurons (DAs) in the substantia nigra pars compacta (SNpc) and the presence of abnormal α-synuclein (α-syn) aggregates (Lewy bodies) are the principal pathological hallmarks of PD3. Misfolded and aggregated α-syn may lead to different pathogenic effects, including induction of oxidative stress4, increase of membrane permeability5, interruption of axonal transport6, synaptic dysfunction7, mitochondrial dysfunction44. A natural product, harmol has fewer side effects and plays an important role in health maintenance and disease control. Finally, natural products can be used as lead compounds for structural modification to synthesize derivatives.
Traditional animal models of PD are designed using toxins, such as 6-hydroxydopamine (6-OHDA), rotenone, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Although toxin-treated models cause the dysfunction and death of dopaminergic neurons within substantia nigra, one of the major drawbacks of traditional models is the lack of Lewy body pathology45. A53T α-syn transgenic mice exhibit a full range of pathology including α-syn aggregation, oligomers, fibrils, phosphorylation, ubiquitination, and progressive neurodegeneration45. In this study, A53T α-syn transgenic animals developed the α-syn inclusions widely distributed throughout each brain region (Fig. 6), and the transgenic mice showed serious motor impairment (Fig. 5). In addition, the internalization and aggregation of misfolded α-syn can lead to ALP dysfunction in cellular and animal models or vice versa, suggesting that a crosstalk between α-syn aggregation and autophagic/lysosomal dysfunction may exist46. Our results prove that the overexpression of α-syn suppresses autophagic/lysosomal function (Figs. 3, 4, 6, and 7). However, the α-syn load did not affect the expression of dopaminergic neuron marker tyrosine hydroxylase (TH) within substantia nigra and striatum (Supplementary Fig. 2). Meanwhile, HPLC (high performance liquid chromatography) analysis showed no significant difference in total striatal DA, HVA (homovanillic acid), and DOPAC (3, 4-dihydroxyphenylacetic acid) content between wild-type and A53T α-syn transgenic mice (Supplementary Fig. 3). Increasing evidence suggests that mice overexpressing A53T α-syn do not lose dopaminergic neurons in substantia nigra, and motor deficits are caused by a loss of brain stem neurons and anterior horn motor neurons of the spinal cord45. In A53T α-syn transgenic mice, dopaminergic neurons do not show the same selective vulnerability that humans demonstrate. Why these neurons have different cross-species characteristics remains unclear, but the lack of neuromelanin formation in mice may be a determinant31. In addition, murine dopaminergic neurons display inexplicable resistance to α-syn-induced neurotoxicity compared with other neuronal populations47.
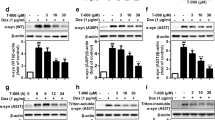
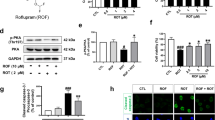
Autophagy and lysosome biogenesis could be enhanced by activating TFEB in vitro and in vivo48. Overexpressing TFEB or inducing its nuclear translocation stimulates ALP functioning and attenuates α-synuclein pathology19. In this study, we identified a natural ALP enhancer, harmol, that promotes the clearance of α-syn in vitro and in vivo (Figs. 1 and 6), and harmol-activated TFEB induces ALP (Figs. 2–4 and 7). AMPK promotes autophagy by inhibiting mTOR in the cytoplasm to allow nuclear translocation of TFEB49. Activation of AMPK-mTOR-TFEB axis-mediated autophagy promotes the clearance of toxic protein aggregates. Indeed, harmol administration increases phosphorylation of AMPK, inhibits phosphorylation of mTOR, and increases the expression of TFEB (Figs. 4 and 7). Furthermore, AMPK has been reported as an activator of autophagy via inhibition of the mTOR complex and direct phosphorylation and activation of ULK1. Specifically, AMPK can promote autophagy by directly activating ULK1 via phosphorylation of Ser317, Ser555, and Ser77750, while mTOR inhibits this process through ULK1 phosphorylation at Ser757. In this study, the p-ULK1(Ser757)/ULK1 is down-regulated, while p-ULK1(Ser317)/ULK1 and p-ULK1(Ser555)/ULK1 are not significantly affected (Figs. 3 and 7). This may indicate that AMPK activation mainly involves mTOR-ULK1 (Ser757) pathway in this study, which regulates autophagy. Compound C/Dorsomorphin is a primary reagent used as an AMPK inhibitor. Compound C can bind to phosphorylated-state mimic T172D mutant kinase domain of the human AMPKα subunit, and compound C can also block the AICAR cellular uptake by competing for adenosine transporter-binding sites51. Inhibition of the AMPK-mTOR-TFEB signaling pathway using AMPK blocker compound C diminishes the effects of harmol on autophagy and α-syn clearance (Fig. 4c–g). Hence, these studies suggest that harmol promotes α-syn clearance via the AMPK-mTOR-TFEB signaling pathway (Fig. 8).
Harmol activates the AMPK and inhibits the mTOR. Cytoplasmic TFEB is highly phosphorylated and located in the lysosomal membrane, which binds with mTOR. Harmol inhibits the mTOR activity and promotes the dephosphorylation of TFEB. Dephosphorylated TFEB translocates into the nucleus, where it binds to the CLEAR sequence, leading to upregulation of the autophagic and lysosomal genes. Eventually, harmol enhances the autophagy-lysosome process and promotes the degradation of α-syn.
Collectively, our research confirms that harmol activates the AMPK-mTOR-TFEB mediated ALP pathway in vitro and in vivo, contributing to the degradation of pathogenic proteins, the restoration of autophagic flux, and lysosomal biogenesis, and the improvement of motor impairment. These results increase the possibility that harmol may be developed into a therapeutic agent against aggregate-prone protein-associated neurodegenerative diseases such as PD, but there are still deficiencies and limitations. It is still not clear how harmol induces AMPK phosphorylation, and future work should aim to identify the upstream molecular signal or target for AMPK activation. Several studies have shown that TFEB also activates AMPK and, in turn, establishes a unique feed-forward loop between TFEB and AMPK52. Thus, harmol may promote autophagy via the APMK-mTOR-TFEB feed-forward loop, and future studies should test this possibility, although harmol may utilize other pathways to degrade α-syn or other mechanisms to regulate ALP.
Methods
Reagents and antibodies
Harmol was purchased from Tokyo Chemical Industry. Doxycycline was purchased from MedChemExpress. Dorsomorphin dihydrochloride was purchased from Targetmol. G418 sulfate was purchased from Amresco. Dopamine hydrochloride, homovanillic acid, 3, 4-dihydroxyphenylacetic acid, tri-sodium citrate dihydrate, citric acid, chloroquine, anti-Flag M2, and anti-α-synuclein antibodies were purchased from Sigma-Aldrich. Anti-TFEB antibody was purchased from Proteintech. Anti-tyrosine hydroxylase, anti-phospho-mTOR (Ser2448), anti-mTOR, anti-phospho-ULK1 (Ser757), anti-phospho-ULK1 (Ser317), anti-phospho-ULK1 (Ser555), anti-ULK1, anti-phospho-AMPKa (Thr172), anti-AMPKa, anti-LAMP1, anti-SQSTM1/p62, anti-TFEB, and anti-CTSD/cathepsin D antibodies were purchased from Cell Signaling Technology. Anti-phospho-α-synuclein and anti-LC3B antibodies were purchased from Abcam. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was purchased from Beyotime Biotechnology.
Animals and treatments
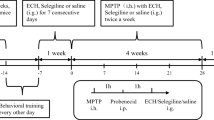
Male hA53T α-syn transgenic mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Animals were propagated and fed at the Technology Industrial Park Development Co., Ltd of Guangzhou University of Chinese Medicine. The mice were housed under a 12 h light-dark cycle at 23 ± 2 °C and 60 ± 15% relative humidity. All animal care and experimental procedures were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of Technology Industrial Park Development Co., Ltd of Guangzhou University of Chinese Medicine, and approved by the committee.
hA53T mice were randomly divided into four groups: saline (n = 6), harmol 10 mg/kg (n = 6), harmol 20 mg/kg (n = 6) and harmol 40 mg/kg (n = 6). Ten-month-old hA53T α-syn transgenic mice were treated with harmol (oral gavage twice daily) or an equal volume of saline for 1 month. Wild-type mice (n = 10) were administrated saline.
Behavioral tests
To evaluate α-syn-induced behavioral deficits, the pole test, rotarod test, open-field test, and automated treadmill gait test was executed. All behavioral studies were double-blinded and repeated three times to obtain the average value for statistical analysis.
Pole test
The climbing-pole test device was a metal rod that was ~50 cm high and 1 cm in diameter, wrapped with gauze to increase friction53. The mice were placed on top of the vertical pole facing down, and the descent time from top to bottom was recorded. Before the test, each mouse was trained for three consecutive days. The experiment was repeated three times in a row on the same day, and the average climbing time was calculated as the evaluation value.
Rotarod test
For the rotarod test, the mice were trained for 3 consecutive days before the actual test54. Mice were placed on an accelerating rotarod cylinder, and the latency time was tested. The speed was slowly accelerated from 4 to 40 rpm within 4 min. The data are presented as the average latency time (three independent trials) on the rotarod.
Open-field test
Animals were placed individually into the center of an acrylic apparatus (50 cm × 50 cm × 40 cm) and allowed to freely explore for 5 min under dim light. The apparatus was cleaned with 70% ethanol solution between each trial to avoid olfactory cues between animals. A video tracking system was used to record the total distance traveled as a measure of autonomous movement55.
Automated treadmill gait test
Treadmill gait assessment was performed with the DigiGait imaging apparatus (Mouse Specifics Inc.)56. Mice were placed on a motorized treadmill within a plexiglass compartment (~25 cm long and 5 cm wide). A camera mounted under the transparent treadmill belt acquired digital video images at 80 frames per second to visualize paw contacts. The treadmill was set at a fixed speed of 15 cm/sec so that most animals could move continuously. Digital videos were analyzed by DigiGait software, which automatically recognized the pawprints, and if necessary, manual changes in the images were made to properly distinguish the pawprints from the background. Then, the images were automatically processed by the software to calculate the values of various gait parameters, including stride frequency, stride width, step angle, absolute paw angle, swing, stride, and stance.
HPLC-ECD analysis
Striatum was homogenized on ice with 0.1 M perchloric acid. After centrifugation at 12,000 × g for 15 min, the supernatant was analyzed using an Agilent HPLC-ECD system (Agilent Technologies Inc., USA) with Agilent Eclipse plus C18 column (4.6 × 100 mm, 3.5 μm). The mobile phase was methanol (10%) and aqueous solution (90%, containing 13.764 g/L citric acid, 10.147 g/L tri-sodium citrate dihydrate, 269.25 mg/L sodium octane sulfonate, 37.2 mg/L ethylenediaminetetraacetic acid disodium salt dehydrate and 149.2 mg/L potassium chloride), and detection was carried out at 35 °C with a flow rate of 0.8 mL/min. For ECD, the detecting voltage was maintained at 750 mV, and the response range of the detector was set at 50 nA. The retention time of 3, 4-dihydroxyphenylacetic acid (DOPAC), dopamine (DA) and homovanillic acid (HVA) was 4.5, 6.2 and 10.3 min, respectively.
Cell culture and drug treatment
N2a cells were provided by Prof. Simon MingYuen Lee (State Key Laboratory of Quality Research in Chinese Medicine and Institute of Chinese Medical Sciences, University of Macau, China) as a gift, and the cells were maintained in DMEM supplemented with 10% FBS. HeLa cells stably expressing 3xFlag-TFEB were supplied by Prof. Ju-**an Song (Guangzhou University of Chinese Medicine, Guangzhou, China) as a gift, and cultured in DMEM containing 10% FBS and 500 μg/mL G418. Inducible PC12 cells were generously gifted from professor Yadong Huang (**an University, Guangzhou, China) and maintained in DMEM supplemented with 5% HS, 10% FBS, and 200 µg/mL G418. For drug treatment, the whole medium was replaced with a fresh medium containing the compounds and then incubated for the indicated periods.
Cell viability
Cells were plated in a 96-well plate for 24 h. After 24 h of compound treatment, cell cytotoxicity was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) analysis, following the manufacturer’s guidelines. Absorbance was measured spectrophotometrically at 570 nm with a microplate reader (Tecan, Switzerland).
High-content screening assay
To quantify TFEB subcellular localization, a high-content assay was performed using HeLa cells stably expressing 3xFlag-TFEB and N2a cells. Cells were seeded in 96-well plates for 24 h and treated with different concentrations of compounds. After 24 h, cells were fixed, permeabilized, blocked, and stained with anti-Flag M2 or anti-TFEB overnight at 4 °C. DAPI, Alexa Fluor®488 (green), or Alexa Fluor® 594 (red) secondary antibodies were added for 2 h at room temperature. Images were acquired by confocal automated microscopy (IN Cell Analyzer 6000, USA). The ratio value of the average nuclear fluorescence intensity divided by the average cytosolic fluorescence intensity was calculated57. Data are represented by the percentage of nuclear translocation at different concentrations of each compound using GraphPad Prism 7 software (GraphPad Software, USA).
Autophagy flux assay
N2a cells were transfected with mCherry-GFP-LC3B adenovirus for 24 h, and then the medium was replaced with a regular culture medium. After 48 h, the cells were respectively treated with harmol, CQ and rapamycin for 2 h. Images were obtained using a fluorescent confocal microscope (Zeiss, Germany) and were quantified by ImageJ software (Version 1.52, USA).
Lyso-Tracker Red staining
Lysosome activity was evaluated using Lyso-Tracker Red (Beyotime Biotechnology, C1046) according to the manufacturer’s protocol. After 24 h of drug treatment, Lyso-Tracker Red (50 nM) was added for 1 h. The cells were washed three times with PBS, and observed using a fluorescent confocal microscope (Zeiss, Germany) and were quantified by ImageJ software (Version 1.52, USA).
Western blotting analysis
Cells or animal tissues were lysed on ice with RIPA lysis buffer containing a protease phenylmethylsulfonyl fluoride and phosphatase inhibitor cocktail. Cytosolic and nuclear fractions were isolated using the Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, 78833) according to the manufacturer’s protocol.
Proteins were separated by 8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After blocking with 5% nonfat milk, the membrane was probed with the primary and secondary antibodies. The protein signals were visualized using an ECL kit and detected by an image reader (GE AI600, USA). All blots were processed in parallel and derive from the same experiment.
Statistics
All experimental data are presented as mean ± standard error of the mean (SEM). Differences among groups (≥3 groups) were analyzed using one-way ANOVA or differences between two groups using Student’s t test by GraphPad Prism 7 software (GraphPad Software, USA). Values of P < 0.05 were considered statistically significant.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Beitz, J. M. Parkinson’s disease: a review. Front. Biosci. 6, 65–74 (2014).
O’Callaghan, C. & Lewis, S. J. G. in International Review of Neurobiology, Vol. 133. (eds K. R. Chaudhuri & N. Titova) 557–583 (Academic Press, 2017).
Wang, Z.-Y. et al. Neuroprotective natural products for the treatment of parkinson’s disease by targeting the autophagy–lysosome pathway: a systematic review. Phytother. Res. 31, 1119–1127 (2017).
Esteves, A. R., Arduíno, D. M., Swerdlow, R. H., Oliveira, C. R. & Cardoso, S. M. Oxidative stress involvement in α-synuclein oligomerization in Parkinson’s disease cybrids. Antioxid. Redox Signal. 11, 439–448 (2008).
Colla, E. et al. Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J. Neurosci. 32, 3301 (2012).
Lamberts, J. T., Hildebrandt, E. N. & Brundin, P. Spreading of α-synuclein in the face of axonal transport deficits in Parkinson’s disease: a speculative synthesis. Neurobiol. Dis. 77, 276–283 (2015).
Volpicelli-Daley, L. A. et al. Exogenous α-synuclein fibrils induce lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011).
Vekrellis, K., **louri, M., Emmanouilidou, E., Rideout, H. J. & Stefanis, L. Pathological roles of α-synuclein in neurological disorders. Lancet Neurol. 10, 1015–1025 (2011).
Lynch-Day, M. A., Mao, K., Wang, K., Zhao, M. & Klionsky, D. J. The role of autophagy in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2, a009357 (2012).
Lindersson, E. et al. Proteasomal inhibition by α-synuclein filaments and oligomers*. J. Biol. Chem. 279, 12924–12934 (2004).
Wang, S.-F., Wu, M.-Y., Cai, C.-Z., Li, M. & Lu, J.-H. Autophagy modulators from traditional Chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J. Ethnopharmacol. 194, 861–876 (2016).
Pan, T., Kondo, S., Le, W. & Jankovic, J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131, 1969–1978 (2008).
Spencer, B. et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in α-synuclein models of Parkinson's and Lewy Body diseases. J. Neurosci. 29, 13578 (2009).
Zhang, K. et al. Targeting autophagy using small-molecule compounds to improve potential therapy of Parkinson’s disease. Acta Pharmaceutica Sin. B 11, 3015–3034 (2021).
Kim, J., Kundu, M., Viollet, B. & Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Bové, J., Martínez-Vicente, M. & Vila, M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat. Rev. Neurosci. 12, 437–452 (2011).
Alers, S., Löffler Antje, S., Wesselborg, S. & Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 32, 2–11 (2012).
Martini-Stoica, H., Xu, Y., Ballabio, A. & Zheng, H. The autophagy–lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neurosci. 39, 221–234 (2016).
Decressac, M. et al. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc. Natl Acad. Sci. 110, E1817 (2013).
Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of parkinson disease: a review. JAMA 323, 548–560 (2020).
Olmedo, G. M. et al. UVA photoactivation of harmol enhances its antifungal activity against the phytopathogens penicillium digitatum and botrytis cinerea. Front. Microbiol. 8, 347 (2017).
Dellal, H. et al. High content screening using new u2os reporter cell models identifies harmol hydrochloride as a selective and competitive antagonist of the androgen receptor. Cells 9, 1469 (2020).
Quintana, V. M. et al. Antiviral activity of natural and synthetic β-carbolines against dengue virus. Antivir. Res. 134, 26–33 (2016).
Moura, D. J., Richter, M. F., Boeira, J. M., Pêgas Henriques, J. A. & Saffi, J. Antioxidant properties of β-carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis 22, 293–302 (2007).
Moura, D. J. et al. Effects of β-carboline alkaloids on the object recognition task in mice. Life Sci. 79, 2099–2104 (2006).
Herraiz, T., González, D., Ancín-Azpilicueta, C., Arán, V. J. & Guillén, H. β-carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 48, 839–845 (2010).
Abe, A. & Kokuba, H. Harmol induces autophagy and subsequent apoptosis in U251MG human glioma cells through the downregulation of survivin. Oncol. Rep. 29, 1333–1342 (2013).
Cui, G. et al. Natural β-carboline alkaloids regulate the PI3K/Akt/mTOR pathway and induce autophagy in insect Sf9 cells. Pestic. Biochem. Physiol. 154, 67–77 (2019).
Cai, C.-Z. et al. Natural alkaloid harmine promotes degradation of alpha-synuclein via PKA-mediated ubiquitin-proteasome system activation. Phytomedicine 61, 152842 (2019).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Giasson, B. I. et al. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34, 521–533 (2002).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Trist, B. G., Hare, D. J. & Double, K. L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 18, e13031 (2019).
Sengul, G., Fu, Y., Yu, Y. & Paxinos, G. Spinal cord projections to the cerebellum in the mouse. Brain Struct. Funct. 220, 2997–3009 (2015).
Carlén, M. What constitutes the prefrontal cortex? Science 358, 478–482 (2017).
Pupyshev, A. B., Korolenko, T. A., Akopyan, A. A., Amstislavskaya, T. G. & Tikhonova, M. A. Suppression of autophagy in the brain of transgenic mice with overexpression of А53Т-mutant α-synuclein as an early event at synucleinopathy progression. Neurosci. Lett. 672, 140–144 (2018).
Charvin, D., Medori, R., Hauser, R. A. & Rascol, O. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat. Rev. Drug Discov. 17, 804–822 (2018).
Devos, D. et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 21, 195–210 (2014).
Mullin, S. et al. Ambroxol for the treatment of patients with parkinson disease with and without glucocerebrosidase gene mutations: a nonrandomized, noncontrolled trial. JAMA Neurol. 77, 427–434 (2020).
West, A. B. Achieving neuroprotection with LRRK2 kinase inhibitors in Parkinson disease. Exp. Neurol. 298, 236–245 (2017).
Jankovic, J. et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti–α-synuclein monoclonal antibody, in patients with parkinson disease: a randomized clinical trial. JAMA Neurol. 75, 1206–1214 (2018).
Zhang, Y. et al. Rapamycin upregulates glutamate transporter and IL-6 expression in astrocytes in a mouse model of Parkinson’s disease. Cell Death Dis. 8, e2611 (2017).
Palma, J.-A. et al. mTOR inhibition with sirolimus in multiple system atrophy: a randomized, double-blind, placebo-controlled futility trial and 1-year biomarker longitudinal analysis. Mov. Disord. 37, 778–789 (2022).
Agostini, F., Masato, A., Bubacco, L. & Bisaglia, M. Metformin repurposing for parkinson disease therapy: opportunities and challenges. Int. J. Mol. Sci. 23, 398 (2022).
Beal, M. F. Parkinson’s disease: a model dilemma. Nature 466, S8–S10 (2010).
Bellomo, G., Paciotti, S., Gatticchi, L. & Parnetti, L. The vicious cycle between α-synuclein aggregation and autophagic-lysosomal dysfunction. Mov. Disord. 35, 34–44 (2020).
Dauer, W. & Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909 (2003).
Song, J.-X. et al. A novel curcumin analog binds to and activates TFEB in vitro and in vivo independent of MTOR inhibition. Autophagy 12, 1372–1389 (2016).
Wu, C. et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int. J. Biol. Sci. 17, 1138–1152 (2021).
Miao, H. et al. PKCα replaces AMPK to regulate mitophagy: Another PEDF role on ischaemic cardioprotection. J. Cell. Mol. Med. 22, 5732–5742 (2018).
Li, Y. & Chen, Y. in Autophagy: Biology and Diseases: Basic Science. (ed. Z.-H. Qin) 85–108 (Springer Singapore, Singapore; 2019).
Fan, Y. et al. Endothelial TFEB (Transcription Factor EB) positively regulates postischemic angiogenesis. Circ. Res. 122, 945–957 (2018).
Wen, J. et al. Tetramethylpyrazine nitrone improves motor dysfunction and pathological manifestations by activating the PGC-1α/Nrf2/HO-1 pathway in ALS mice. Neuropharmacology 182, 108380 (2021).
Tarantini, S. et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J. Cereb. Blood Flow. Metab. 35, 1871–1881 (2015).
Kim, S. et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103, 627–641.e627 (2019).
Amende, I. et al. Gait dynamics in mouse models of Parkinson’s disease and Huntington’s disease. J. Neuroeng. Rehabilitation 2, 20 (2005).
Settembre, C. et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 (2012).
Acknowledgements
This research is funded by the National Natural Science Foundation of China (Nos. U1801287, 81872842, 82073821, 82073712, and 82061160374), the Natural Science Foundation of Guangdong Province (2020A1515011061), the Science and Technology Planning Project of Guangdong Province (Nos. 2021A0505080012, 2020B1111110004 and 2018B020207008), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (No. 2017BT01Y036), and the Science and Technology Planning Project of Guangzhou City (No. 20212210005).
Author information
Authors and Affiliations
Contributions
Z.J.Z. and J.X. designed the study. J.X. drafted the manuscript. Z.J.Z., X.Q.Z., and C.H.H. revised the manuscript. Y.L.A. and G.L.Z. contributed to analysis of data. X.B.S., W.C., and Y.Q.W. contributed to resources. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, J., Ao, YL., Huang, C. et al. Harmol promotes α-synuclein degradation and improves motor impairment in Parkinson’s models via regulating autophagy-lysosome pathway. npj Parkinsons Dis. 8, 100 (2022). https://doi.org/10.1038/s41531-022-00361-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-022-00361-4
- Springer Nature Limited