Abstract
Polymer-based pure organic room-temperature phosphorescent materials have tremendous advantages in applications owing to their low cost, vast resources, and easy processability. However, designing polymer-based room-temperature phosphorescent materials with large Stokes shifts as key requirements in biocompatibility and environmental-friendly performance is still challenging. By generating charge transfer states as the gangplank from singlet excited states to triplet states in doped organic molecules, we find a host molecule (pyrrolidone) that affords charge transfer with doped guest molecules, and excellent polymer-based organic room-temperature phosphorescent materials can be easily fabricated when polymerizing the host molecule. By adding polyaromatic hydrocarbon molecules as electron-donor in polyvinylpyrrolidone, efficient intersystem crossing and tunable phosphorescent from green to near-infrared can be achieved, with maximum phosphorescence wavelength and lifetime up to 757 nm and 3850 ms, respectively. These doped polyvinylpyrrolidone materials have good photoactivation properties, recyclability, advanced data encryption, and anti-counterfeiting. This reported design strategy paves the way for the design of polyvinylpyrrolidone-based room-temperature phosphorescent materials.
Similar content being viewed by others
Introduction
Luminescent materials are of significance for both commercial and scientific purposes1,2,3,4,5,6,7,8,9,10,38,39,40 have been proposed to obtain ORTP. However, most of the ORTP materials reported to date, exhibit only green and yellow phosphorescent emission, in contrast, white or red long-persistent emission is rarely reported1,2. Moreover, the phosphorescence lifetimes of the reported red ORTP materials are very short, probably resulting from the energy gap law, limiting the practical applications. To establish ORTP materials with long-wavelength emission necessarily comes from materials with low energy level triplet state (T1). Lower T1 brings two major issues to phosphorescence. One is that lower T1 implies a larger band gap (ΔEst) between S1 and T1, which is not favorable to intersystem crossing (ISC) of excitons, making it difficult to observe phosphorescence. The other is that lower T1 is more likely to lead to non-radiative depletion of excitons, which results in a significant reduction in the lifetime and intensity of the phosphorescence.
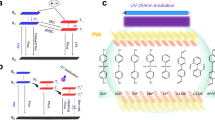
Fortunately, an energy transfer passage could be unlocked by generating intermolecular charge transfer states as an energy bridge, and usually, T1 could be stabilized in doped systems. In our previous study, the efficient red long-persistent emission from purely organic doped molecules was obtained41. These materials can be easily fabricated by mixing pyrene (electron donor) and benzophenone (electron acceptor) using the melt-casting method. The mechanism study shows that it is the charge-transfer (CT) states that facilitate the ISC of the guest, resulting in room-temperature phosphorescence (RTP) from guest triplet states (Fig. 1a). This study gives us the hint that to make good use of polyaromatic hydrocarbons as the guests with very low T1 energy levels due to the highly conjugated nature of these molecules, which is crucial for long-wavelength phosphorescence. But at the same time, they also have very high S1 energy levels, which will result in a very large ∆Est, and ISC is difficult to occur. The above problems can be easily solved by constructing the host-guest charge transfer states, thus ORTP materials with long-wavelength emission can be prepared. Practically, the poor reproducibility and flexibility of crystal-based ORTP materials limits their applications. It is well known that polymer-based afterglow materials show good flexibility and transparency6,42. Moreover, polymer-based ORTP materials are suitable for large-scale production through solution processing which is beneficial for commercialization19,22. To avoid the cumbersome preparation process of modifying polymers with electron donor and acceptor groups to achieve good luminescence43, it is necessary to apply a doped host-guest strategy in the field of polymer-based ORTP materials by constructing materials with aromatic hydrocarbons as the guests (electron donor) and appropriate polymer as the host (electron acceptor) (Fig. 1b).
a The ORTP mechanism of the host-guest system in organic crystals was proposed by our group in a previous work. b Strategy used in this work for constructing polymer-based ultralong ORTP materials. c Phosphorescence performance distribution of polymer-based ORTP materials. Abs. is Absorption, Flu. Is Fluorescence, Non-rad. is Non-irradition, Pho. is Phosphorescence.
Polymer-based pure ORTP materials have become a rapidly growing research field, and various preparation strategies have been developed, such as do**44,45,46, copolymerization47,48,49, homopolymerization50,51,52, and host–guest inclusion53,54,55. However, the overall performance of RTP, including phosphorescence lifetime and quantum efficiency, still needs to be further optimized. More to the point, only sporadic examples have emitted ultra-long wavelength afterglows so far56,57,58, and most of them are organic crystals1,2,59,60,61. It is still very challenging to achieve polymer-based pure ORTP materials with long wavelength emission.
In this work, we constructed the tunable luminescent polymer-based ORTP materials by using highly conjugated polyaromatic hydrocarbons (PAHs) molecules as guests and polyvinylpyrrolidone (PVP) as the host. Materials with a series of phosphorescence wavelengths from 550 nm to 757 nm and lifetimes from 139 ms to 3850 ms can be prepared (Fig. 1c). In addition, these polymer-based ORTP materials show excellent photoactivation properties and thus can be used as programmable labels to achieve dual fluorescent-phosphorescent advanced anti-counterfeiting functions.
Results
First of all, when different fluorescent PAHs molecules as guests (Fig. 2a) and PVP (Mw = 40,000-45,000 g/mol) as host are mixed at a mass ratio of 1% to prepare doped films, and fluorescence emission under 365 nm UV excitation is observed (Fig. 2b UV on and Supplementary Fig. 1). Compared to a blank PVP film with irradiation by 365 nm UV for a short time (Supplementary Fig. 2), intense different color afterglows from green to NIR are manifested after irradiation for a longer time (Fig. 2b UV off and Supplementary movie 1-10). As can be seen in Fig. 2c, the RTP of these polymer-based materials is gradually enhanced when increasing UV irradiation time and their maximum emission peaks are listed in Table 1. Moreover, the luminescent lifetimes gradually increase with longer irradiation time (Fig. 2d). In the meantime, good to excellent phosphorescence quantum yields (Φp) of these activated films are determined and listed in Table 1.
a Molecular structures of the host and guest molecules. b Fluorescence and phosphorescence images of PVP films doped with the different guest molecules under UV light at 365 nm, with the name of the guests at the top of the image and the duration of the afterglow observable to the naked eye at the bottom of the image. c Delayed emission spectra of these doped PVP films after photoactivation under UV irradiation at 365 nm at different times, delay time: 1 ms. d Emission decay curves of the doped PVP films before and after photoactivation.
In addition, the delayed emission spectra of PAHs doped PVP films (Fig. 2c) are consistent with the delayed emission spectra of PAHs in a dilute solution of 2-methyltetrahydrofuran at 77 K (Supplementary Fig. 3). These results suggest that the doped materials emit light from the triple excited state of the guest molecules which are dispersed in the polymer (PVP). Although the PAHs are potential phosphorescent luminogens, PVP the host is necessary for the efficient phosphorescence of PAHs. Generally, the host material can act as a sealer to prevent the triplet excitons from being quenched by oxygen48. Therefore, we investigated the influence of O2 on the luminescent behavior of BecPh/PVP and BePh/PVP films and found that the RTP was further enhanced under vacuum conditions (Supplementary Fig. 4). The quenching of the phosphorescence of the pristine samples without photoactivation may be caused by the energy transfer from the triplet excitons of the guest compound to the O2 molecule in the PVP. These results also indicate that the luminescence involves triplet states and explain the results in Figs. 2c and 2d that the luminescent intensities and lifetimes are enhanced along with the increase of irradiation time allowing O2 consumption. In addition, four annealing temperatures, 20 °C, 40 °C, 60 °C and 80 °C were selected for thermal analysis of the doped films. As the annealing temperature increases, the required annealing time decreases, which is in accordance with the time-temperature equivalence principle. In order to achieve faster annealing results, an annealing temperature of 80 °C and an annealing time of 5 min were chosen. These results fully demonstrate that these doped films possess satisfactory photoactivated RTP performance.
In order to clarify what promotes the luminescent switch from fluorescence to phosphorescence for doped PAHs, ultrafast femtosecond transient absorption (fs-TA) spectra are employed to investigate the distinct luminescence behaviors of the photo-activated polymer film doped with Py and BePe (Fig. 3). When using 365 nm as a pump beam, the guest is excited, and the excitation of PVP can be mostly avoided (Supplementary Fig. 5). Figure 3a shows two obvious excited state absorption (ESA) peaks for Py dispersed in PMMA. The band at 470 nm is ascribed to the absorption from the excited singlet state and the peak at 510 nm is assigned to the absorption of Py’s excimer. The fs-TA spectra of Py dispersed in PMMA are very close to that in MeCN solution (Supplementary Fig. 6). A dramatic difference can be seen for Py-doped PVP film. For the CT between host and guest in Py/PVP doped films, the population and transformation of the CT absorption is usually very fast. As shown in (Supplementary Fig. 7), the transient absorption peaks gradually rise up and have a red-shift from 433 to 441 nm for Py/PVP film and from 521 to 525 nm for BePe/PVP film at the beginning delay times. These steps are attributed to the CT process in the doped film. Generally speaking, the host is the vast majority, and the guest content is very small in the doped systems. Therefore, it is usually very difficult to observe the cationic or anionic radicals by the transient absorption when it undergoes intermolecular charge transfer in the excited state. But in our system, the conjugation of guest molecules is very good and aromatic, thus the cationic radical (441 nm) of the guest is directly observed by fs-TA. However, the host molecule is not conjugated, thus the molar extinction coefficient of its anionic radicals will be relatively small, and it is difficult to detect the corresponding anionic cations. Nevertheless, the detection of the cationic radical signal of the guest molecule in the transient absorption spectra can further prove that the intermolecular charge transfer process in the excited state has occurred between host and guest in the doped systems. After 72.2 ps, an ESA peak at 441 nm obviously shows up (Fig. 3b and Supplementary Fig. 8). Based on our previous study, this 441 nm peak is assigned to the absorption of the Py radical cation41. Subsequently, the long-lived triplet state with the main ESA peak around 370 nm is generated along with the decay of 441 nm and 470 nm absorption peaks62,63. Finally, the transient species decays gradually to zero. From the kinetics fitting, the 370 nm peak for Py-doped PVP film decays much slower than that for Py dispersed in PMMA (Supplementary Fig. 9), which is consistent with the phenomenon that Py-doped PVP film shows RTP feature. More convincingly, similar results are observed in BePe/PVP films. For BePe dispersed in PMMA (Fig. 3c), the peak at 422 nm is ascribed to the absorption from the excited singlet state of BePe, and the band at 514 nm is assigned to the absorption of BePe’s excimer. The fs-TA spectra of BePe dispersed in PMMA are very close to the spectral evolution of BePe in the dichloromethane solvent (Supplementary Fig. 10). When BePe is dispersed into PVP, the fs-TA spectra show a new ESA peak at 525 nm (Fig. 3d), which is attributed to the absorption of the BePe radical cation. This can also prove the intermolecular charge transfer from BePe molecules to side groups of PVP. In addition, BecPh/PVP and DBeCh/PVP films are also explored by fs-TA spectra (Supplementary Fig. 11), which are similar to those of Py/PVP and BePe/PVP. All these results show that it is charge transfer states facilitating the ISC processes of the PAHs/PVP.
To further confirm the intermolecular CT process between the PAHs and PVP polymer, theoretical calculations are carried out based on density functional theory (DFT) at the B3LYP/6-31 G(d) level. For this do** system, the host is a polymer. Since it is very difficult to calculate and analyze the molecular orbital energy level of polymer, we select a monomer (1-vinyl-2-pyrrolidinone (VP)) of PVP as the template for analysis. The results show that VP has higher highest occupied molecular orbital (HOMO) and lower lowest unoccupied molecular orbital (LUMO) energy levels than PVP. As the unit number of VP increases, the HOMO of the VP combinations gradually increases while the LUMO gradually decreases (Fig. 4a). The energy differences between the HOMOs of the guests and LUMO of PVP could be small enough for intermolecular charge transfer64,65 (Supplementary Fig. 12). Since the LUMOs of the guests have higher energy than the LUMO of PVP, charge transfer can occur from the excited guests to PVP after 365 nm UV lighting. To validate the above experimental data, the excited state calculations have been performed on the guests and the pair. The adiabatic energy maps can be drawn based on the optimized geometric structure of the singlet and triplet excited states, and the spin-orbit coupling (SOC) constants are calculated based on the optimized S1.
The calculated results show that the ∆ES1→T1 values of the guests are large, and the SOC values are quite small (Table 2), showing that ISC is difficult to occur. This result is consistent with the spectroscopic results that the guest molecules are fluorescent molecules rather than phosphorescent ones. But when the fs-TA spectra of such molecules were tested, anomalies were found. Taking the fs-TA spectra of Py and BePe as an example, the characteristic absorption peak of the excited triplet state of Py at 411 nm in MeCN and the excited triplet state of BePe at 470 nm for BePe in DCM can be detected. In addition, similar results were obtained for other PAHs molecules when fs-TA experiments were performed. This implies that PAHs molecules can actually undergo ISC to produce the corresponding excited triplet states, which is completely inconsistent with the calculated results. Through literature review66,67,68,69,70 and further experiments, we have revealed the reason why PAHs can undergo ISC. For PAHs molecules, whose structures are all planar, they are very prone to generate the excimers upon excitation, which allows them to undergo ISC, leading to the generation of the corresponding triplet excitons. To test the hypothesis, we have measured the fs-TA spectra of Py with different concentrations in MeCN. The results found that when the concentration of Py is very small, the transient absorption signal of excited triplet state excitons of Py is very small because it is very difficult to form the excimers. However, with the increasing concentration of Py, it is more likely to produce excimers, so the intensity of the characteristic absorption peak of Py’s excimer (508 nm) obviously increases, and the characteristic absorption peak of excited triplet states (411 nm) of Py is also obviously enhanced (Supplementary Fig. 13a). In addition, we also tested the fs-TA spectra of BePe with different concentrations in DCM, and the same results can be obtained (Supplementary Fig. 13b). But even so, the formation of the excimers based on the conjugated molecule promotes the ISC process only to a small extent, which explains the fact that most of these guests show very weak RTP when doped with polymers such as PVA, PMMA, etc. For example, the doped film of Py/PMMA produces a very weak red phosphorescence. However, when excited state intermolecular charge transfer is formed between the host and PAHs molecules, the ISC process of PAHs molecules is greatly facilitated, resulting in the generation of a large number of triplet excitons. This can be verified not only from the small-molecule do** systems that we have previously studied41. But more importantly, the triplet state yield of the guest PAHs in the polymer system can also be dramatically boosted by its intermolecular charge transfer with the side groups of the PVP. This makes the RTP of Py/PVP doped films much stronger than that of Py/PMMA doped films at the same do** ratio (Supplementary Fig. 14). The calculated ΔEst and SOC values of the complex satisfy the optimal conditions for the ISC (Fig. 4b and Supplementary Table 1). Therefore, the charge transfer state opens a new trajectory for the ISC of the guests, which will dramatically improve the ISC process of PAHs guest molecules. More importantly, the calculated triplet state energies essentially match the phosphorescence colors, which also confirms that the phosphorescence emission originates from the guest triplet state.
Based on the luminescent phenomena and theoretical studies, the RTP mechanism of these doped films is becoming clear. As shown in Fig. 4c, when the polymer side groups and guest molecules form a unit, the charge transfer from guests to PVP side groups is facilitated. When guests are excited, a charge transfer state is formed in a short time. This host-stabilized charge transfer state can transform into a triplet charge transfer state, and then give a triplet state for guests through charge recombination. This is comparable to facilitating the ISC process of the guest molecules, resulting in a substantial increase in the number of guest triplet state excitons. Eventually, as the photoactivation time is prolonged, the O2 in the PVP is completely consumed, and RTP occurs after removing the excitation light (Fig. 4d).
It is well known that PVP has shown good prospects in the fields of medicine, food, and cosmetics due to its excellent solubility and biocompatibility. After do** with PAHs, it still exhibits excellent performance. If PVP solutions with different guests are applied as coatings on transparent plastic paper, it is difficult for the naked eye to see the written pattern clearly after the solvent evaporates. However, under UV irradiation, the patterns written on the material show a corresponding strong fluorescence. After 2 min of irradiation, the UV light is removed, and the patterns are drawn with corresponding afterglows. (Fig. 5a and Supplementary Fig. 15). In addition, this material can be used for the manufacturing of 3D anti-counterfeiting objects. These 3D objects appear transparent in sunlight and exhibit the same luminescent properties as before after removing excitation (Fig. 5b).
a Luminescence images of various patterns made from the polymer-based ORTP materials as coatings under UV lamp (365 nm) excitation and after removing the UV light (the names of the relevant guests are labeled at the top of the images). b Luminescent images of 3D casting objects of Py/PVP and FlAn/PVP under daylight, on UV lamp (365 nm) excitation, and after removal of UV light. c Schematic of writing, reading, and erasing procedure of programmable label. d Luminescent images of programmable labels based on Py/PVP and FlAn/PVP film.
Moreover, the activation-deactivation process is repeatable because there is no significant change in the RTP spectrum, luminescent intensity, and lifetime (Supplementary Fig. 16). Inspired by the switchable RTP and steady-state luminescence characteristics, the Py/PVP and FlAn/PVP are exploited as an erasable transparent film for the Programmable Label. Figure 5c shows the overview of the writing, reading, and erasing procedure of the programmable label. By mask illumination of the samples, any pattern can be applied as phosphorescent information storage. Py/PVP and FlAn/PVP films are covered with a mask A of the Chinese character ‘鑫’ and then irradiated by the 365 nm UV light (9 mWcm-2) for 120 seconds. Remove the mask after the writing is completed, and the corresponding afterglow of the Chinese character ‘鑫’ appears on the doped film when the information is read. It is worth noting that this pattern is invisible to the naked eye under ambient conditions. Under the excitation of 365 nm UV light, the white Chinese character ‘鑫’ can be observed on both FlAn/PVP and Py/PVP films. Moreover, the resulting picture could be retained for several seconds due to the RTP properties of FlAn/PVP and Py/PVP. When ceasing the excitation light source, FlAn/PVP and Py/PVP display yellow and red written patterns, respectively, and the background turns black. Afterward, the pattern is erased via annealing at 80 °C for 5 min, and a renewed film is obtained by replacing mask A with mask B of quick response code and repeating the above procedures, the quick response code can be clearly printed. Interestingly, using the mobile phone to scan the patterns highlighted by the yellow and red RTP can facilely access the webpage information (Fig. 5d and Supplementary Fig. 17). In order to further enhance the application prospects of these materials, Py/PVP and FlAn/PVP solutions are directly coated on paper. After the solvent has evaporated, the previous operation is repeated. When the excitation light source is removed, clear writing patterns can still be obtained (Supplementary Fig. 18).
Discussion
In summary, we have innovatively developed a strategy to assist PAHs that lack any significant means of SOC for the ISC process to achieve efficient ORTP by using the construction of charge transfer states with PVP polymer. Specifically, ORTP from green to near-infrared is easily achieved by do** different PAHs in PVP. Among them, the RTP emissions of Py/PVP, BeAn/PVP, DBeCh/PVP, and BePe/PVP materials are all in the red region with RTP lifetimes of 358, 139, 468 and 214 ms, and afterglow durations of 4, 3, 7 and 4 s, respectively, which are very promising red RTP emitters with exceptional performance. The good RTP property is attributed, from spectroscopy and computational studies, to intermolecular charge transfer (from PAHs to side groups of PVP), which opens a new gangplank to promote the ISC process of PAHs that leads to the emission from excited triplet states of PAHs. More importantly, these high-performance ORTP materials with different colors show great potential in applications from information storage and data encryption to programmable labels and bioimaging. Our study further complements the insufficiencies in the interpretation of the ORTP mechanism in the polymer-based do** system and serves as a guideline for the future design of large Stokes shift ORTP materials with long wavelength and lifetime emission.
Methods
Material science
Polyvinylpyrrolidone (PVP), Benzo[c]phenanthrene (BecPh, 95%), Benzo[a]phenanthrene (BePh, 99%), Pyrene (Py, 98%), Fluoranthene (FlAn, 98%), Benz[a]anthracene (BeAn, 98%), Picene (Pi, 99%), Dibenz[a,h]anthracene (BeTe, 98%), Dibenzo[g,p]chrysene (DBeCh, 98%), Benzo[ghi]perylene (BePe, 98%), and Coronene (Co, >94%) were obtained from J&K Scientific Ltd. All other solvents and reagents were purchased with analytical grade and used without further purification.
Preparation of the polymer-based RTP film
Precursor solution synthesized by mixing different polyaromatic hydrocarbons (PAHs) as the guests and the PVP as host according to the mass ratio of 1:100 in organic solution. The prepared solution was added dropwise on the quartz sheet, and the corresponding polymer-based RTP films could be obtained after all the solvent evaporated.
UV-vis absorption experiments
The UV-Vis absorption spectra of samples were recorded by a spectrometer (PE Lambda950) from PERKINELMER company.
Photoluminescence (PL) experiments
The PL spectra were recorded by a spectrometer (FLS980) from Edinburgh Instruments. Doped films are used for all tests, and the amounts of samples for PL measurement are similar. Time-resolved PL decay kinetics and PL spectra were recorded in room temperature by the FLS980 from Edinburgh company.
Femtosecond transient absorption (fs-TA) experiments
The fs-TA experiments were carried out by employing a femtosecond regenerative amplified Ti: sapphire laser system in which the amplifier was seeded with the 84 fs laser pulses from an oscillator laser system. The probe pulse was generated by ~5% of the amplified 800 nm laser pulses to produce a white-light continuum (350–800 nm) in a CaF2 crystal. After that this probe laser was split into two parts before pathing the sample. One probe laser went through the sample while the other probe laser went to the reference spectrometer in order to monitor the fluctuations of the probe laser intensity. The laser 365 nm with 0.2 mW power was used as the excitation wavelength. The single-wavelength dynamics fitting was performed based on equation (1). The τ is referred to as delay time, and τ0 is zero time. The τp and A are the instrument response value and the proportion of species, respectively.
Theoretical calculations
The theoretical calculations are carried out using the Gaussian 16 software packages. B3LYP is selected as the function, and 6-31 g(d) is selected as the bases set to optimize the structures and analyze the energy level, the cartesian coordinates for the complexes (VP, (VP)2, (VP)5, (VP)10, BecPh, BePh, FlAn, Py, BeAn, Pi, BeTe, DBeCh, BePe, Co) calculated in this study were put in the Supplementary Data 1. The structures of the host-guest complexes are shown in Multiwfn software.
Data availability
The authors declare that the data supporting the results of this study are available within the article and its Supplementary Information. Extra data are available from the corresponding authors on request.
References
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Yan, X. et al. Recent advances on host–guest material systems toward organic room temperature phosphorescence. Small 18, 2104073 (2022).
Kabe, R. & Adachi, C. Organic long persistent luminescence. Nature 550, 384–387 (2017).
Gao, H. & Ma, X. Back Cover: Recent progress on pure organic room temperature phosphorescent polymers. Aggregate 2, e116 (2021).
Chen, C. et al. Carbazole isomers induce ultralong organic phosphorescence. Nat. Mater. 20, 175–180 (2021).
Wang, J., Lou, X.-Y., Wang, Y., Tang, J. & Yang, Y.-W. Recent advances of polymer‐based pure organic room temperature phosphorescent materials. Macromol. Rapid Commun. 42, 2100021 (2021).
Li, M.-D. et al. Dynamics of oxygen-independent photocleavage of blebbistatin as a one-photon blue or two-photon near-infrared light-gated hydroxyl radical photocage. J. Am. Chem. Soc. 140, 15957–15968 (2018).
Li, D. et al. Completely aqueous processable stimulus responsive organic room temperature phosphorescence materials with tunable afterglow color. Nat. Commun. 13, 347 (2022).
Wang, Z. et al. Accessing excitation- and time-responsive afterglows from aqueous processable amorphous polymer films through do** and energy transfer. Adv. Mater. 34, 2202182 (2022).
Wei, J. et al. Induction of strong long‐lived room‐temperature phosphorescence of N‐phenyl‐2‐naphthylamine molecules by confinement in a crystalline dibromobiphenyl matrix. Angew. Chem. Int. Ed. 55, 15589–15593 (2016).
**e, Z. et al. Wide-range lifetime-tunable and responsive ultralong organic phosphorescent multi-host/guest system. Nat. Commun. 12, 3522 (2021).
Wang, X. et al. TADF‐type organic afterglow. Angew. Chem. Int. Ed. 60, 17138–17147 (2021).
Chen, B. et al. An organic host–guest system producing room‐temperature phosphorescence at the parts‐per‐billion level. Angew. Chem. Int. Ed. 60, 16970–16973 (2021).
Wang, Y. et al. Förster resonance energy transfer: an efficient way to develop stimulus-responsive room-temperature phosphorescence materials and their applications. Matter 3, 449–463 (2020).
Lei, Y. et al. Efficient and organic host–guest room-temperature phosphorescence: tunable triplet–singlet crossing and theoretical calculations for molecular packing. Chem. Sci. 12, 6518–6525 (2021).
Chen, J. et al. Asymmetric diarylamine guests for a host–guest system with stimulus-responsive room temperature phosphorescence. J. Mater. Chem. C. 11, 6290–6295 (2023).
Dai, W. et al. Controllable modulation of efficient phosphorescence through dynamic metal‐ligand coordination for reversible anti‐counterfeiting printing of thermal development. Adv. Funct. Mater. 33, 2210102 (2023).
Bian, L. et al. Simultaneously enhancing efficiency and lifetime of ultralong organic phosphorescence materials by molecular self-assembly. J. Am. Chem. Soc. 140, 10734–10739 (2018).
Su, Y. et al. Ultralong room temperature phosphorescence from amorphous organic materials toward confidential information encryption and decryption. Sci. Adv. 4, eaas9732 (2018).
Kwon, M. S., Lee, D., Seo, S., Jung, J. & Kim, J. Tailoring intermolecular interactions for efficient room‐temperature phosphorescence from purely organic materials in amorphous polymer matrices. Angew. Chem. Int. Ed. 53, 11177–11181 (2014).
Daly, M. L., Kerr, C., DeRosa, C. A. & Fraser, C. L. Meta-alkoxy-substituted difluoroboron dibenzoylmethane complexes as environment-sensitive materials. Acs Appl. Mater. Inter. 9, 32008–32017 (2017).
Zhang, Y. et al. Large-area, flexible, transparent, and long-lived polymer-based phosphorescence films. J. Am. Chem. Soc. 143, 13675–13685 (2021).
Li, G. et al. High‐efficiency thermally activated delayed fluorescence materials via a shamrock‐shaped design strategy to enable OLEDs with external quantum efficiency over 38%. Aggregate 4, e382 (2023).
Zhang, K. Y. et al. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev. 118, 1770–1839 (2018).
Yang, J. et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat. Commun. 9, 840 (2018).
He, Z. et al. Achieving persistent, efficient, and robust room‐temperature phosphorescence from pure organics for versatile applications. Adv. Mater. 31, 1807222 (2019).
Wang, X. et al. Organic phosphors with bright triplet excitons for efficient X-ray-excited luminescence. Nat. Photon. 15, 187–192 (2021).
Wang, Y. et al. High performance of simple organic phosphorescence host–guest materials and their application in time‐resolved bioimaging. Adv. Mater. 33, 2007811 (2021).
**ao, F. et al. Guest-host doped strategy for constructing ultralong-lifetime near-infrared organic phosphorescence materials for bioimaging. Nat. Commun. 13, 186 (2022).
Bolton, O., Lee, K., Kim, H.-J., Lin, K. Y. & Kim, J. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 3, 205–210 (2011).
Hamzehpoor, E. & Perepichka, D. F. Inside back cover: crystal engineering of room temperature phosphorescence in organic solids (Angew. Chem. Int. Ed. 25/2020). Angew. Chem. Int. Ed. 59, 10196 (2020).
An, Z. et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 14, 685–690 (2015).
Wang, Z., Zhu, C.-Y., Wei, Z.-W., Fan, Y.-N. & Pan, M. Breathing-ignited long persistent luminescence in a resilient metal–organic framework. Chem. Mater. 32, 841–848 (2020).
Huang, G. et al. Long‐range charge transportation induced organic host–guest dual color long persistent luminescence. Adv. Opt. Mater. 9, 2101337 (2021).
Baroncini, M., Bergamini, G. & Ceroni, P. Rigidification or interaction-induced phosphorescence of organic molecules. Chem. Commun. 53, 2081–2093 (2017).
Gao, R., Kodaimati, M. S. & Yan, D. Recent advances in persistent luminescence based on molecular hybrid materials. Chem. Soc. Rev. 50, 5564–5589 (2021).
He, Z. et al. White light emission from a single organic molecule with dual phosphorescence at room temperature. Nat. Commun. 8, 416 (2017).
Cai, S. et al. Enhancing ultralong organic phosphorescence by effective π-type halogen bonding. Adv. Funct. Mater. 28, 1705045 (2018).
Yang, Y. et al. Efficient and color‐tunable dual‐mode afterglow from large-area and flexible polymer-based transparent films for anti-counterfeiting and information encryption. Angew. Chem. Int. Ed. 134, e202201820 (2022).
Zheng, X. et al. A processable, scalable, and stable full-color ultralong afterglow system based on heteroatom-free hydrocarbon doped polymers. Mater. Horiz. 10, 197–208 (2023).
Yang, G. et al. Construction and application of large stokes-shift organic room temperature phosphorescence materials by intermolecular charge transfer. J. Phys. Chem. Lett. 14, 6927–6934 (2023).
Zhang, Y. et al. Room temperature phosphorescent (RTP) thermoplastic elastomers with dual and variable RTP emission, photo-patterning memory effect, and dynamic deformation RTP response. Adv. Sci. 9, 2103402 (2022).
Wang, D. et al. Employing lactam copolymerization strategy to effectively achieve pure organic room-temperature phosphorescence in amorphous state. Adv. Opt. Mater. 7, 1901277 (2019).
Al-Attar, H. A. & Monkman, A. P. Room-temperature phosphorescence from films of isolated water-soluble conjugated polymers in hydrogen-bonded matrices. Adv. Funct. Mater. 22, 3824–3832 (2012).
Lee, D. et al. Room temperature phosphorescence of metal-free organic materials in amorphous polymer matrices. J. Am. Chem. Soc. 135, 6325–6329 (2013).
Su, Y. et al. Excitation-dependent long-life luminescent polymeric systems under ambient conditions. Angew. Chem. Int. Ed. 59, 9967 (2020).
Gu, L. et al. Color-tunable ultralong organic room temperature phosphorescence from a multicomponent copolymer. Nat. Commun. 11, 944 (2020).
Ma, X., Xu, C., Wang, J. & Tian, H. Amorphous pure organic polymers for heavy-atom-free efficient room-temperature phosphorescence emission. Angew. Chem. Int. Ed. 57, 10854 (2018).
Lin, X., Wang, J., Ding, B., Ma, X. & Tian, H. Tunable-emission amorphous room-temperature phosphorescent polymers based on thermoreversible dynamic covalent bonds. Angew. Chem. Int. Ed. 60, 3459 (2021).
Ogoshi, T. et al. Ultralong room-temperature phosphorescence from amorphous polymer poly(styrene sulfonic acid) in air in the dry solid state. Adv. Funct. Mater. 28, 1707369 (2018).
Zhou, Q. et al. Emission mechanism understanding and tunable persistent room temperature phosphorescence of amorphous nonaromatic polymers. Mater. Chem. Front. 3, 257–264 (2019).
Zhu, J.-C. et al. The design, synthesis and order-enhanced emission of luminescent liquid crystalline polymers based on a “jacketing” effect. Polym. Chem. 10, 6342–6349 (2019).
Li, J.-J., Zhang, H.-Y., Zhang, Y., Zhou, W.-L. & Liu, Y. Room-temperature phosphorescence and reversible white light switch based on a cyclodextrin polypseudorotaxane xerogel. Adv. Opt. Mater. 7, 1900589 (2019).
Chen, H., Ma, X., Wu, S. & Tian, H. A rapidly self-healing supramolecular polymer hydrogel with photostimulated room-temperature phosphorescence responsiveness. Angew. Chem. Int. Ed. 53, 14149–14152 (2014).
Zhang, Z.-Y. et al. A Synergistic Enhancement Strategy for Realizing Ultralong and Efficient Room-Temperature Phosphorescence. Angew. Chem. Int. Ed. 59, 18748 (2020).
Kong, L. et al. Tunable ultralong multicolor and near-infrared emission from polyacrylic acid-based room temperature phosphorescence materials by FRET. Chem. Eng. J. 469, 143931 (2023).
Lin, F. et al. Stepwise energy transfer: near-infrared persistent luminescence from doped polymeric systems. Adv. Mater. 34, 2108333 (2022).
Zhu, Y. et al. Achieving color-tunable persistent afterglow from ultralong polyacrylamide-based room-temperature phosphorescence materials through phosphorescence Förster resonance energy transfer. Eur. Polym. J. 202, 112600 (2024).
Si, Y. et al. Organic host-guest materials with bright red room-temperature phosphorescence for persistent bioimaging. Chin. J. Chem. 41, 1575–1582 (2023).
Fateminia, S. M. A. et al. Organic nanocrystals with bright red persistent room-temperature phosphorescence for biological applications. Angew. Chem. Int. Ed. 56, 12160 (2017).
Zhu, T., Yang, T., Zhang, Q. & Yuan, W. Z. Clustering and halogen effects enabled red/near-infrared room temperature phosphorescence from aliphatic cyclic imides. Nat. Commun. 13, 2658 (2022).
Badger, B. & Brocklehurst, B. Absorption spectra of dimer cations. Part 3.—Naphthalene and anthracene derivatives and pyrene. Trans. Faraday Soc. 65, 2588–2594 (1969).
Mori, Y., Shinoda, H., Nakano, T. & Kitagawa, T. Formation and decay behaviors of laser-induced transient species from pyrene derivatives 1. spectral discrimination and decay mechanisms in aqueous solution. J. Phys. Chem. A 106, 11743–11749 (2002).
Gould, I. R., Young, R. H., Mueller, L. J., Albrecht, A. C. & Farid, S. Electronic structures of exciplexes and excited charge-transfer complexes. J. Am. Chem. Soc. 116, 8188–8199 (1994).
Jenekhe, S. A. & Osaheni, J. A. Excimers and exciplexes of conjugated polymers. Sinence 265, 765–768 (1994).
Rodgers, M. A. J. Formation kinetics of the pyrene dimer cation observed by pulse radiolysis. Chem. Phys. Lett. 9, 107–108 (1971).
Kira, A., Arai, S. & Imamura, M. Pyrene Dimer Cation as Studied by Pulse Radiolysis. J. Chem. Phys. 54, 4890–4895 (1971).
Kryachko, E. S. Dicationic states of benzene dimer: Benzene dimer cation and benzene dication parenthood patterns. Int. J. Quantum Chem. 107, 2741–2755 (2007).
Getoff, N., Solar, S., Richter, U.-B. & Haenel, M. W. Pulse radiolysis of pyrene in aprotic polar organic solvents: simultaneous formation of pyrene radical cations and radical anions. Radiat. Phys. Chem. 66, 207–214 (2003).
Kira, A., Nakamura, T. & Imamura, M. Pyrene—naphthalene complex radical cations. Chem. Phys. Lett. 54, 582–584 (1978).
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (22173055 awarded to L.D., 22273057 awarded to M.D.L.), Innovation Team Project (2019KCXTD007 awarded to M.D.L.) of the Educational Commission of Guangdong Province of China, the Universities Joint Laboratory of Guangdong, Hong Kong and Macao (2021LSYS009 awarded to M.D.L.) and the Natural Science Foundation of Guangdong Province (2022A1515011661 awarded to M.D.L., 2023A1515012631 awarded to L.D.).
Author information
Authors and Affiliations
Contributions
G.X.Y. conceived the study, synthesized the materials, and performed RTP mechanism and application studies. L.D. supervised the project. G.X.Y., S. B.H., X.D., X.L.S., W.H., B.S., L.D., and M.D.L. discussed the results and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, G., Hao, S., Deng, X. et al. Efficient intersystem crossing and tunable ultralong organic room-temperature phosphorescence via do** polyvinylpyrrolidone with polyaromatic hydrocarbons. Nat Commun 15, 4674 (2024). https://doi.org/10.1038/s41467-024-48913-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48913-x
- Springer Nature Limited