Abstract
Ae. aegypti mosquitoes transmit some of the most important human viral diseases that are responsible for a significant public health burden worldwide. The small interfering RNA (siRNA) pathway is considered the major antiviral defense system in insects. Here we show that siRNA pathway disruption by CRISPR/Cas9-based Ago2 knockout impaired the mosquitoes’ ability to degrade arbovirus RNA leading to hyper-infection accompanied by cell lysis and tissue damage. Ago2 disruption impaired DNA repair mechanisms and the autophagy pathway by altering histone abundance. This compromised DNA repair and removal of damaged cellular organelles and dysfunctional aggregates promoted mosquito death. We also report that hyper-infection of Ago2 knockout mosquitoes stimulated a broad-spectrum antiviral immunity, including apoptosis, which may counteract infection. Taken together, our studies reveal novel roles for Ago2 in protecting mosquitoes from arbovirus infection and associated death.
Similar content being viewed by others
Introduction
The yellow fever mosquito Aedes aegypti is the principal vector of numerous medically important human pathogens, including dengue virus (DENV), Zika virus (ZIKV), chikungunya virus (CHIKV) and Mayaro virus (MAYV). These pathogens are transmitted between humans and mosquitoes and represent a major public health causing socioeconomical burden globally1,2. The populations at risk of mosquito-borne diseases have grown substantially in recent years and could potentially extend to low-risk countries in Asia, Europe and North America because of the spread of Aedes mosquito vectors as a result of climate change3,4,5. Current methods to control mosquito-borne diseases that have emerged, or re-emerged, in many geographical regions are proving insufficient because of insecticide resistance and a lack of effective vaccines. Vector control remains the principal method for controlling and limiting mosquito-borne diseases6. In recent years, with the development of genome editing and mosquito transgenesis, novel control strategies based on population suppression or population replacement have been proposed as alternatives for the control of mosquito-borne diseases7.
To defend against infection with arboviruses, mosquitoes use their innate immune system, which includes the small interfering RNA (siRNA) pathway, Toll pathway, JAK/STAT pathway, and other defensesFull size image
Since the biogenesis of vsRNA was not affected by Ago2 disruption, we investigated whether the number of 21 nt viral small interfering RNAs (vsiRNAs) that bound to siRNA pathway factors was altered in Ago2−/− mutants. A higher number of MAYV vsiRNA was observed in Ago2−/− mutants as compared to WT mosquitoes (Fig. 2d), but the ratio of vsiRNA reads to the total vsRNA reads was lower in Ago2−/− mutants (Fig. 2e) because they produced a greater number of non-21nt-vsRNA as compared to WT mosquitoes (Fig. 2b). There was a similar distribution of 21 nt vsiRNA in the positive or negative strand of the MAYV genome between Ago2−/− mutants and WT mosquitoes, but the relative abundances of the vsiRNAs were different because of the higher vsRNA reads in Ago2−/− mutants (Fig. 2f). Therefore, our results show that Ago2 disruption does not affect the production of arboviral sRNAs but does impair the ability of the siRNA pathway to degrade viral RNAs, as reflected by a high virus titer and an impaired RNA silencing in Ago2−/− mutants.
The total sRNAs were also mapped to the Ae. aegypti genome. The results showed that Ago2 disruption significantly reduced the abundance of the mosquito sRNAs at 4 dpi with MAYV (Fig. 2g), likely because of a higher fraction of MAYV sRNAs in the total sRNAs in Ago2−/− mutants (Fig. 2h). As compared to WT mosquitoes, Ago2−/− mutants produced a higher number of mosquito 21 nt siRNAs (Fig. 2i), suggesting that Ago2 disruption may increase the abundance of the mosquito endogenous dsRNAs. The length distribution of the mosquito sRNAs showed no difference between Ago2−/− mutants and WT mosquitoes, and both showed two peaks at 21–23 and 26–30 nt, corresponding to microRNAs and piwiRNAs, respectively (Fig. 2j).
To further determine the influence of the Ago2 disruption on the mosquito’s ability to degrade arbovirus RNA, we performed independent sRNA sequencing on another Ago2 mutant line (ArgoN−/−) and its parental line (Cas9) at 2 dpi and 7 dpi with DENV2. The sRNA sequencing results of DENV2-infected ArgoN−/− mutants were very similar to what we observed in MAYV-infected Ago2−/− mutants, including a significantly higher number of total DENV2 sRNA (Supplementary Fig. 4c, d, e), 21nt DENV2 siRNA (Supplementary Fig. 4f, g), total mosquito sRNA (Supplementary Fig. 4h), and 21 nt mosquito siRNA reads (Supplementary Fig. 4i), and no difference in length distribution of the mosquito sRNAs (Supplementary Fig. 4j) in ArgoN−/− mutants as compared to the Cas9 mosquitoes, and the high yield of DENV2 vsRNAs could be resulted from the high virus titer in ArgoN−/− mutants (Supplementary Fig. 4k).
Overall, our studies have shown that Ago2 disruption does not affect the biogenesis of sRNAs in either arboviruses or mosquitoes and the increased abundance of viral sRNAs that we found is due to the high arbovirus titer in Ago2-deficient mosquitoes.
Arbovirus infection results in high mortality of Ago2-deficient mosquitoes through cell lysis
To determine whether hyper-infection of arboviruses can lead to the death of Ago2−/− mutants, we performed a mortality assay involving DENV2, ZIKV, and MAYV infections transmitted through oral blood-feeding as well as through direct injection of viruses into the mosquito hemolymph. Our results showed that Ago2−/− mutants experienced a significantly higher mortality upon infection with the three different arboviruses than did WT mosquitoes infected through the two different infection routes, but direct injection of the viruses resulted in higher mortality than did natural infection through blood-feeding (Fig. 3a). Of all the arboviruses we tested, MAYV infection resulted in the highest mortality of Ago2−/− mutants (Supplementary Fig. 5a), and the median survival after MAYV infection was 6 days for the orally infected and 4 days for the virus-injected Ago2−/− mutants (Fig. 3a); therefore, Ago2−/− mutants and MAYV infection were used for the future experiments to explore the mechanism of arbovirus-induced mortality of Ago2−/− mutants. Arbovirus infection also caused the mortality of ArgoN−/− mutants, a mutation in the ArgoN domain of Ago2, but it is lower than that of Ago2−/− mutants (Supplementary Fig. 5b); these results were correlated with the virus titer in the various Ago2 knockout mutant lines (Fig. 1d), suggesting that the high mortality of the Ago2−/− mutants may be a result of their high infection intensity. The virus infection intensity-dependent mortality was then confirmed through injection of different titers of MAYV into Ago2−/− mutants where a higher titer of MAYV led to higher mortality of Ago2−/− mutants (Supplementary Fig. 5c).
a survival curves of Ago2−/− and wild-type (WT) females that ingested a blood meal containing DENV2, ZIKV, or MAYV or were mock-infected (given C6/36 cell culture medium), or were injected with DENV2, ZIKV, or MAYV or mock-infected medium. P values between Ago2−/− mutants and WT mosquitoes were determined using the logrank (Mantel-Cox) test. Each experiment comprised at least three biological replicates. The error bands were indicated by the shaded region. IFA detection with the anti-MAYV monoclonal antibody (green) of MAYV in midguts (b) and salivary glands (c) of Ago2−/− and WT females at various days post-infection (dpi). Nuclei were stained with DAPI (blue). The zoomed areas are indicated as white boxes and arrows. White arrows indicate abnormal nuclei, and red arrows indicate strong fluorescence with a “ball” shape. Ultrastructural TEM images showing cross-sections of midgut tissue of Ago2−/− mutants and WT mosquitoes at 4 dpi (d) and 7 dpi (e) with MAYV. Yellow arrows indicate individual virion; white dotted lines outline a cluster of virions. Scale bars are indicated on each image. Abbreviations: BL basal lamina, nc nucleus. The IFA and TEM images in (b–e) are representative of three biologically independent samples. Source data are provided as a Source Data file.
We also used IFA to examine the infection of the midguts and salivary glands of Ago2−/− mutants and WT mosquitoes after MAYV and DENV2 infection. At 4 dpi with MAYV, the midguts of the Ago2−/− females showed a strong fluorescence with a “ball”-like shape that comprises hyper-infected midgut cells, indicating that the insects were hyper-infected by viruses (Fig. 3b), and the nuclei of a large proportion of the midgut cells were highly condensed and exhibited an abnormal shape when compared to those of the WT midguts (Fig. 3b); these findings suggested that the cells of Ago2−/− mutants were likely undergoing lysis as a consequence of virus hyper-infection. At 7 dpi with MAYV, the infection and lysis of midgut epithelial cells were even higher in Ago2−/− females, and most of the midgut cells were lysed (Fig. 3b). At 7 dpi and 14 dpi with DENV2, the higher infection and lysis of the midgut epithelial cells were also observed in Ago2−/− females when compared to the WT midguts (Supplementary Fig. 5d). A similar pattern of condensed nuclei and hyper-infection was observed in the salivary glands of Ago2−/− females upon MAYV infection (Fig. 3c), suggesting that cell lysis might occur in multiple tissue and cell types in Ago2−/− mutants in response to arbovirus infection. We did not observe lysis of midguts and salivary glands in uninfected Ago2−/− mutants as compared to the WT mosquitoes (Supplementary Fig. 5e). The virus -induced lysis of salivary gland cells is likely to have affected the shedding of viral particles into the saliva, leading to the observed reduction in feeding and arboviral transmission by the Ago2−/− mutants (Fig. 1h, j).
We then employed transmission electron microscopy (TEM) to examine the cell lysis in the midguts of Ago2−/− females upon arbovirus infection. Ultrastructural studies showed that the nuclei of the Ago2−/− midgut cells displayed an elongated shape, and the nucleolus was not well formed at 4 dpi with MAYV, compared to the WT midgut cells (Fig. 3d), indicating that the cells may undergo nuclear fragmentation and apoptosis. Moreover, clusters of virions were observed in the midgut cells of Ago2−/− mutants, whereas the virions were evenly distributed in the WT midguts. At 7 dpi with MAYV, the pattern of infection in the WT midguts was similar to that at 4 dpi, but more virions were observed; in contrast, the midgut epithelial cells of Ago2−/− females were completely lysed and devoid of nuclei, and the virus particles appeared to be present throughout the entire cell (Fig. 3e).
In summary, our data demonstrate that Ago2−/− mutants have a weakened defense against arbovirus infection and that hyper-infection lyses the mutant mosquitoes’ cells and damages their tissues, leading to pathogenesis that compromises their feeding propensity (Fig. 1h) and viability (Fig. 3a).
Arbovirus hyper-infection stimulates broad-spectrum antiviral immunity in Ago2-deficient mosquitoes
To probe to the underlying mechanism governing the infection-induced mortality in Ago2−/− mutants, we compared the whole transcriptomes of MAYV-infected Ago2−/− and WT females, as well as uninfected Ago2−/− and WT females. As compared to the WT mosquitoes, we found that 1,279 genes were upregulated, and 1,128 genes were downregulated in Ago2−/− mutants in response to MAYV infection at 4 dpi (Fig. 4a and Supplementary Data 1), and 555 upregulated and 280 downregulated genes in uninfected Ago2−/− mutants (Supplementary Data 2).
a flowchart illustrating the design and outcome of RNAseq experiments comparing transcriptome changes in Ago2−/− mutants and WT mosquitoes. DE genes, differentially expressed genes. n = 3 biological replicates. b interaction network illustrating the upregulated gene families in Ago2−/− mutants. The light color indicates the small P-value (one-sided Fisher’s exact test), and the small size of the bubble indicates the few genes in the GO term. The immunity- and defense-related GOs are indicated by asterisks. c fold enrichment of the significantly upregulated gene families in Ago2−/− mutants. The immunity-related gene families are outlined red. d heatmap illustrating transcriptome changes in the upregulated immunity genes in Ago2−/− mutants. e detection of caspase activity in midguts of Ago2−/− and WT females. Data are presented as mean ± SD (n = 4). f confirmation by qPCR of the upregulated expression of caspase and apoptosis genes in midguts of Ago2−/− females. Data are presented as mean ± SD (n = 4). g TUNEL assay to detect apoptotic cells (red) in midguts of Ago2−/− and WT females. MAYV antigen is indicated by green, and nuclei are in blue. h survival curves for the MAYV-infected WT females that were fed with apoptosis inducer PAC-1 or apoptosis inhibitor Z-VAD-FMK (VAD) or an equal amount of DMSO in 10% sucrose solution. Data are presented as percents ± SE (n = 3). i virus titer in WT females treated with PAC-1 or VAD at 7 dpi with MAYV. Data were presented as box and whiskers (Min to Max), n = 30. j upregulated immune genes related to B-cell, T-cell, complement (Suchi), or macroglobulin (Mg) in Ago2−/− mutants. k upregulated immunoglobulin (Ig)-like genes in Ago2−/− mutants. P values were determined by one-way ANOVA (e and f), an unpaired two-sided Mann-Whitney test (i), or a logrank (Mantel-Cox) test (h). Source data are provided as a Source Data file.
GO and interacting network analysis of the upregulated genes showed an enrichment of mRNAs corresponding to genes with predicted functions in the categories of immune response, defense, and response to stimuli in Ago2−/− mutants at 4 dpi with MAYV when compared to the WT mosquitoes (Fig. 4b and Supplementary Table 1). A group of immune genes, including caspases, C-type lectin (CTL), and leucine-rich repeat (LRR) family members was significantly enriched in Ago2−/− mutants (Fig. 4c and Supplementary Data 1). Most of the upregulated immune genes such as genes from the CTL, fibrinogen-related protein (FREP), Clip-domain serine protease (CLIP) and peptidoglycan recognition protein (PGRP) family have immune-defense/antibacterial functions, but two groups of genes (apoptosis genes and Dcr2) have specific antiviral functions, and four groups including genes from the IMD, Toll, JAK/STAT and JNK pathway have both antibacterial and antiviral functions (Fig. 4d). There are four groups of genes with unknown functions that encode proteins with immunoglobulin (Ig)-like, macroglobulin (Mg) domain, Sushi/SCR/CCP domain, and T/B cell-related features. We also found a large number of putative immune genes with predicate antibacterial function that were significantly upregulated in uninfected Ago2−/− mutants as compared to WT mosquitoes at 4 days post blood feeding (Supplementary Fig. 6a and Supplementary Data 2), suggesting these groups of genes may not be induced by viral hyper-infection.
At least eight caspase and apoptosis-related genes were upregulated, and one inhibitor of apoptosis gene (IAP4) was downregulated in Ago2−/− mutants at 4 dpi with MAYV (Supplementary Fig. 6b). The upregulated genes included 6 caspase genes (CASPS8, CASPS9, CASPS17, CASPS18, CASPS19, and Dronc), IMP-like IAP-antagonist Micheob_x-like protein (IMP-like, AAEL014196) and nuclear apoptosis-inducing factor 1 (NAIF1, AAEL022840) genes. However, only one caspase gene (CASPS8) was significantly upregulated in uninfected Ago2−/− mutants as compared to WT mosquitoes (Supplementary Data 2). These data point to an upregulation of the apoptosis pathway in Ago2−/− mutants in response to MAYV infection. Next, we measured overall caspase activity of Ago2−/− and WT females using a caspase-3 assay kit, and our results showed that caspase activity was increased in the midguts and carcasses of Ago2−/− mutants at 4 dpi with MAYV (Fig. 4e and Supplementary Fig. 6c) and in DENV2-infected Ago2−/− mutants at 14 dpi (Supplementary Fig. 6d) when compared to WT mosquitoes. Transcript abundance analyses with qPCR confirmed that the expression of caspase and IMP-like genes was significantly induced in the midguts and carcasses of Ago2−/− females at 4 dpi with MAYV (Fig. 4f and Supplementary Fig. 6e) and in DENV2-infected Ago2−/− females at 14 dpi (Supplementary Fig. 6f). As compared to the WT mosquitoes, the midguts of the Ago2−/− mutants had more apoptotic cells (by TUNEL staining) (Fig. 4g and Supplementary Fig. 6g). The Ago2−/− females exhibited a lower and higher probability of survival after MAYV infection (Fig. 4h) when treated with an apoptosis inhibitor (Z-VAD-FMK) or inducer (PAC-1), respectively, and the virus titer was significantly increased or reduced in the inhibitor- or inducer-treated females (Fig. 4i). These results together demonstrate that apoptosis is stimulated in Ago2−/− mutants upon arbovirus infection, and these mutants may use an alternative antiviral mechanism against arboviral infection.
Another group of upregulated immune genes have a predicted function related to the mammalian immune system, including B/T cell-mediated humoral/cellular immunity and complement. These genes were highly induced in Ago2−/− mutants at 4 dpi with MAYV (Fig. 4j–k).
Overall, this stimulation of broad-spectrum antiviral genes in Ago2−/− mutants upon arbovirus infection suggests that mosquitoes may engage other antiviral defense mechanisms to protect themselves from viral infection when the major antiviral siRNA pathway has been disrupted.
Ago2 disruption impairs DNA repair and replication and reduces histone abundance
GO and interacting network analyses of the downregulated genes showed that genes with functions related to DNA replication, repair, and metabolism were significantly downregulated in Ago2−/− mutants at 4 dpi with MAYV (Fig. 5a and Supplementary Table 2), indicating that the DNA replication and repair mechanisms are significantly impaired in Ago2−/− mutants. Then, we compared the expression of genes with predicated function in DNA damage response (DDR) that controls lesion detection and DNA repair27 between Ago2−/− mutants and WT mosquitoes. We found that most of the DDR genes that are involved in base excision repair (BER), double-strand break (DSB) repair, DNA mismatch repair (DMR) and nucleotide excision repair (NER) were downregulated in Ago2−/− mutants in response to MAYV infection at 4 dpi (Fig. 5b, c and Supplementary Data 1), as compared to the WT mosquitoes. DMR and NER are the largest groups of the downregulated DNA repair genes. Most DDR genes were also downregulated and 8 of them were significantly downregulated in uninfected Ago2−/− mutants as compared to WT mosquitoes at 4 days post blood feeding (Supplementary Fig. 7a and Supplementary Data 2), suggesting Ago2 disruption may have a direct impact on DNA repair. The downregulation of DDR genes was further confirmed by qPCR in Ago2−/− females with or without MAYV infection at 4 dpi (Fig. 5d), and with or without DENV2 infection at 14 dpi (Supplementary Fig. 7b), as compared to the WT controls.
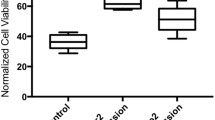
a interaction network illustrating downregulated gene families in Ago2−/− mutants. The light color indicates small P-value (one-sided Fisher’s exact test), and the small size indicates few genes in the GO term. GOs related to DNA repair and replication are indicated by asterisks. b volcano plot illustrating fold changes in DNA repair genes (DRGs) in Ago2−/− mutants. c heatmap illustrating transcriptome changes in upregulated DRGs in Ago2−/− mutants. d qPCR detecting expression of downregulated DRGs in Ago2−/− carcasses. e fold enrichment of downregulated gene families in Ago2−/− mutants. f downregulated histone genes in MAYV infected Ago2−/− mutants. g volcano plot illustrating fold changes of histones-related genes in Ago2−/− mutants. h overall histone level in MAYV infected Ago2−/− and WT mosquitoes. i qPCR detecting expression of histone genes in carcasses of Ago2−/− and WT mosquitoes at 4 days post-infection (n = 4). j virus titer in histone dsRNA-injected WT females at 7 dpi as determined by plaque assay. Left: n = 35 (dsGFP), n = 28 (dsHis2A), n = 15 (dsHis3) and n = 26 (dsHis4). Righ: n = 29 (dsGFP) and n = 51 (dsHis). k virus titer in WT mosquitoes treated with histone deacetylase inhibitor Scriptaid (Scr), n = 20. l survival curves of MAYV-infected WT females that were fed with Scriptaid or DMSO in 10% sucrose solution. Data are represented as percents ± SE (n = 3). qPCR detecting DRG expression (m) and histone expression (n) in WT females that were injected with dsHis (mixture of dsRNAs of His2A, His3 and His4) or dsDRG (mixture of dsRNAs of cdk1, RAD50, APE1 and KPNA2) at 3- and 7-days post injection, respectively. n = 4. P values were determined by a one-way ANOVA (d, i), an unpaired two-sided t-test (h), two-way ANOVA with multiple comparisons (m and n), a logrank (Mantel-Cox) test (l), or an unpaired two-sided Mann-Whitney test (j, k). Data are presented as mean ± SEM (h, i, m and n), and box and whiskers (Min to Max) (d and j) from 4 replicates. Source data are provided as a Source Data file.
Taken together with our observation from the midgut TEM and TUNEL studies that the nuclei of the midgut cells in the mutant mosquitoes had an abnormal shape (Fig. 3d, e) and underwent apoptosis and DNA fragmentation (Fig. 4g and Supplementary Fig. 6g), our results here suggest that hyper-infection of arboviruses induces DNA damage in Ago2−/− mutants, and Ago2 disruption impairs DNA repair mechanisms.
Several histone genes were among those downregulated in Ago2−/− mutants (Fig. 5e), and the expression of at least 20 histone genes was significantly reduced by more than 2-fold in Ago2−/− mutants at 4 dpi with MAYV (Fig. 5f). Next, we compared the mRNA abundance of histones and histone-modifying enzyme genes between Ago2−/− mutants and WT mosquitoes. Our results showed that the majority of these genes were downregulated in MAYV infected (Fig. 5g and Supplementary Data 1) and uninfected Ago2−/− mutants (Supplementary Fig. 7c, d and Supplementary Data 2), and a reduction of up to 36.6% at 4 dpi with MAYV (Fig. 5h) and 24.59% without infection (Supplementary Fig. 7e), suggesting that impact of Ago2 disruption on histone abundance is independent of viral infection and downregulation of histones may be responsible for the impaired DNA replication and repair mechanisms in Ago2−/− mutants. Next, we used qPCR to examine the expression of three histone core genes (His2A, His3 and His4) that were significantly downregulated in the transcriptome of MAYV infected Ago2−/− mutants. Our results showed that the mRNA abundance of these histone genes was significantly decreased in the midguts and carcasses of Ago2−/− females, with or without MAYV infection (Fig. 5i and Supplementary Fig. 7f), and with or without DENV2 infection (Supplementary Fig. 7g), when compared to the corresponding WT mosquitoes, indicating that Ago2 disruption affects the expression of histones, independent of arbovirus infection.
MAYV infection reduced the expression of histone genes in carcasses and midguts of the WT mosquitoes when compared to non-infected mosquitoes (Fig. 5i and Supplementary Fig. 7g), suggesting that MAYV infection affects histone expression. To better understand the interaction between histones and arbovirus infection, we silenced three histone genes (His2A, His3, and His4) by injecting dsRNAs into the WT mosquitoes prior to virus infection. When compared to the GFP control, silencing of each histone gene significantly reduced the MAYV titer (Fig. 5j). When the three histone genes were co-silenced, a significant 2.75-fold (P < 0.0001) reduction in the MAYV titer was observed (Fig. 5j). Furthermore, when the WT mosquitoes were treated with a histone deacetylase (HDAC) inhibitor (Scriptaid), which inhibits HDAC to remove acetyl groups from the lysine residues of histone and HDAC inhibitor treatment increases genome-wide histone acetylation28, they exhibited a significantly higher MAYV titer (Fig. 5k) and lower survival rate (Fig. 5l) than did those mosquitoes treated with a DMSO control. These data indicate that silencing of histones results in a decreased virus titer.
To address whether histone downregulation leads to the impaired DDR in Ago2−/− mutants, we co-silenced three histone genes (His2A, His3, and His4) in WT mosquitoes and then assayed mRNA abundance of DNA repair genes. Our results show that silencing of histone genes significantly affects mRNA abundance of all the DNA repair genes at 3 days post dsRNA injection and 7 of 8 DNA repair genes at 7 days post dsRNA injection (Fig. 5m). On the other hand, co-silencing of DNA repair genes (cdk1, RAD50, APE1 and KPNA2) reduces the mRNA abundance of His3 at 3 days post dsRNA injection but does not affect mRNA abundance of all three histone genes at 7 days post dsRNA injection even though silencing of DNA repair genes persists at that time point (Fig. 5n and Supplementary Fig. 8a). Then, we silenced individual DNA repair gene and investigated its impact on histone gene mRNA abundance. We found that silencing of cdk1 had the most effect on histone gene mRNA abundance at 3 days post dsRNA injection (Supplementary Fig. 8b). At 7 days post dsRNA injection, gene silencing was only persistent in RAD50 dsRNA-injected mosquitoes, but its silencing increased expression of His2A and His3 (Supplementary Fig. 8c). Hence, the effect of DNA repair gene silencing on histone gene expression is inconclusive. Taken together, our data support the hypothesis that histone gene downregulation is the major reason for the observed defects in DNA replication and repair mechanisms in Ago2−/− mutants.
In summary, Ago2 disruption reduces the production of histones and compromises the DNA replication and repair mechanism in the arboviruses-infected mosquitoes. The downregulation of histone genes may play a protective role during arbovirus infection, likely through impairing the replication mechanism of the arboviruses.
Ago2 disruption represses autophagy by altering histone abundance
We found that Ref(2)P (polyubiquitin binding protein p62), which serves as a marker of autophagic flux in Drosophila29,30, was significantly upregulated, and two autophagy-related genes (Atg) were downregulated in the transcriptomes of Ago2−/− mutants upon MAYV infection (Fig. 6a). Most autophagy-related genes were also downregulated in uninfected Ago2−/− mutants and one of them (Atg10) was strongly downregulated (Supplementary Fig. 9a and Supplementary Data 2). Then, we used qPCR to examine the expression of a panel of Atg (Atg5, Atg7, Atg10, and Atg12), p62 and P53, a transcription factor that transactivates a panel of Atg genes in Drosophila31, between Ago2-/- mutants and WT mosquitoes in response to infection with different arboviruses. Our results confirmed the downregulation of Atg and P53 in Ago2−/− mutants with or without MAYV infection at 4 dpi (Fig. 6b), and with or without DENV2 infection at 14 dpi (Supplementary Fig. 9b), as compared to the WT controls. We also observed downregulation of p62 in uninfected Ago2−/− mutants as compared to the WT mosquitoes and MAYV infection increased p62 expression in Ago2−/− mutants (Fig. 6b). The protein levels of ATG8 and phosphatidylethanolamine (PE) of ATG8 (ATG8-PE), and P53 were also reduced in Ago2−/− mutants as compared to the WT controls (Fig. 6c and Supplementary Fig. 9c–f). These data suggest a less active autophagy pathway in Ago2−/− mutants.
a upregulated p62 and downregulated autophagy-related genes (Atg) in the transcriptome of Ago2−/− mutants. n = 3. b expression of p62, P53 and Atg genes in the carcasses of Ago2−/− and WT females at 4 days post-infection (dpi) with MAYV, as detected by qPCR. n = 4 biological replicates. c western blot showing protein levels of ATG8, ATG8-phosphatidylethanolamine (ATG8-PE) and P53 in Ago2−/− and WT females at 4 dpi with MAYV; β-actin was used as a loading control. Western blots were repeated at least three times, and representative results are shown. Lysotracker Red (LTR) staining (d) showing the impairment of autophagy and LTR integrated density (e) in midguts of Ago2−/− females at 4 dpi with MAYV. Nuclei were stained with DAPI (blue). n = 6 biologically independent samples. Autolysosomes (arrowheads) and autophagosomes (white arrows) were detected by TEM in the midguts of Ago2−/− and WT females at 4 dpi (f) and 7 dpi (g) with MAYV. Yellow arrows indicate virions, and yellow arrowheads indicate cell debris. Abbreviations: Mi mitochondria, nc nucleus. n = 3 biologically independent samples. h virus titer in P53- or p62- dsRNA-injected WT females at 10 dpi with MAYV as determined by plaque assay. dsRNA for GFP was injected as a control. Data were presented as box and whiskers (Min to Max), n = 41 (dsGFP), n = 40 (dsP53 and dsp62). i survival curves of the MAYV-infected WT females that were fed with autophagy inhibitor 3-MA or inducer rapamycin (RAPA) or an equal amount of DMSO in 10% sucrose solution. Data were presented as percents ± SE (n = 3). j qPCR detecting the expression of a panel of Atg genes in histone dsRNA-injected WT mosquitoes at 3 days post-injection and 4 days post-blood-feeding. n = 4 biological replicates. P values were determined by an unpaired two-sided t-test (a, e and j), one-way ANOVA for (b), an unpaired two-sided Mann-Whitney test for (h), and a logrank (Mantel-Cox) test (i). Data are represented as mean ± SEM (a and b). Source data are provided as a Source Data file.
To further confirm the repressed autophagy in Ago2−/− mutants, we used Lysotracker Red (LTR), a vital dye used for the detection of acidic organelles (including autolysosomes), to stain the midguts from Ago2−/− and WT females at 4 dpi with MAYV. As compared to the WT mosquitoes, in which numerous LTR-positive dots accumulated in the midgut epithelium, the midguts of Ago2−/− females showed a strongly reduced accumulation of LTR-positive cells (Fig. 6d) and LTR integrated density (Fig. 6e) upon MAYV infection. TEM was further used to document autophagy defects in the midgut cells of Ago2−/− females. At 4 dpi with MAYV, many autophagosomes and autolysosomes were observed in the midgut cells of the WT mosquitoes, whereas the midgut cells of Ago2−/− mutants exhibited a strong reduction in these autophagic structures, as well as numerous areas representing black cell debris/aggregates (Fig. 6f). A large number of autophagosomes and autolysosomes were also present in the midgut cells of the WT mosquitoes at 7 dpi with MAYV (Fig. 6g). Because of the strong cell lysis induced in Ago2−/− mutants at 7 dpi with MAYV, we saw no autophagosomes or autolysosomes, but cell debris and black aggregates were present in both the cytoplasm and the nuclei (Fig. 6g). Taken together, these results demonstrate that Ago2 disruption severely impairs the induction of autophagy upon arbovirus infection.
To confirm the role of autophagy in inhibiting arbovirus infection in mosquitoes, we silenced P53 and p62 in the WT mosquitoes prior to infection and determined the virus titer in the silenced mosquitoes. The results showed that the MAYV titer was significantly elevated in the P53-silenced mosquitoes and suppressed in the p62-silenced mosquitoes (Fig. 6h). When Ago2−/− mutants were treated with the autophagy inhibitor 3-MA or an inducer, rapamycin, they exhibited a significantly lower or higher probability of survival, respectively, upon MAYV infection (Fig. 6i). These data indicate that autophagy plays a role in preventing mosquito death during arbovirus infection by inhibiting viral infection.
The regulation of autophagy is linked to histone modification and altered histone abundance in mammals32,33. To test the effect of histone loss on autophagy in mosquitoes, we silenced three histone genes (His2A, His3, and His4) by injecting a mixture of dsRNAs for these genes into the WT mosquitoes and monitoring the Atg expression by qPCR in the silenced mosquitoes. As compared to the GFP control, silencing the histone genes significantly reduced the expression of a panel of autophagy genes (Atg5, Atg7, Atg10, and Atg12) in both sugar-fed and blood-fed mosquitoes (Fig. 6j), and these results were correlated with the silencing efficiency of the histone genes (Supplementary Fig. 9g). As compared to the sugar-fed mosquitoes, the blood-fed mosquitoes exhibited a significantly higher expression of Atg5 and Atg10 in the GFP control mosquitoes, but not in the histone-silenced mosquitoes.
In summary, Ago2 disruption significantly repressed the autophagy pathway in mosquitoes, most likely by affecting histone production, and this reduced autophagy promoted mosquito death by hampering the removal of damaged cell aggregates in the arbovirus-hyper-infected mosquitoes.
A proposed model of arbovirus-induced mosquito death mechanism
We propose a working model to summarize our findings and to explain an arbovirus-induced mosquito death mechanism in Ago2−/− mutants (Fig. 7): (1) Ago2 disruption does not affect the biogenesis of small RNA but impairs the arboviral RNA degradation through a defective siRNA pathway in Ae. aegypti; (2) Ago2 disruption leads to arbovirus hyper-infection of mosquitoes, which is accompanied by cell lysis and results in mortality; (3) Ago2 disruption influences the production of histones, which represses the autophagy and DNA repair mechanism in arboviruses-hyper-infected mosquitoes. (4) hyper-infection by arboviruses and defect in DNA repair along with cytoplasmic degradation promote mosquito death.