Abstract
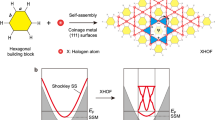
Kagome-lattice materials possess attractive properties for quantum computing applications, but their synthesis remains challenging. Herein, based on the compelling identification of the two cleavable surfaces of Co3Sn2S2, we show surface kagome electronic states (SKESs) on a Sn-terminated triangular Co3Sn2S2 surface. Such SKESs are imprinted by vertical p-d electronic hybridization between the surface Sn (subsurface S) atoms and the buried Co kagome-lattice network in the Co3Sn layer under the surface. Owing to the subsequent lateral hybridization of the Sn and S atoms in a corner-sharing manner, the kagome symmetry and topological electronic properties of the Co3Sn layer is proximate to the Sn surface. The SKESs and both hybridizations were verified via qPlus non-contact atomic force microscopy (nc-AFM) and density functional theory calculations. The construction of SKESs with tunable properties can be achieved by the atomic substitution of surface Sn (subsurface S) with other group III-V elements (Se or Te), which was demonstrated theoretically. This work exhibits the powerful capacity of nc-AFM in characterizing localized topological states and reveals the strategy for synthesis of large-area transition-metal-based kagome-lattice materials using conventional surface deposition techniques.
Similar content being viewed by others
Introduction
Transition metal (TM)-based kagome materials provide an attractive platform for investigating correlated topological properties1,2,3,4,5,6,7,8,9,10,11,12,13,14,15 and develo** kagome-lattice applications16. However, the number of already synthesized kagome materials is limited, thus the construction of tailored kagome electronic band structures remains challenging12. Kagome physics is generally two-dimensional (2D) but kagome electronic states (KESs) are usually induced by kagome planes embedded in the bulk material. These planes of most known TM-based kagome materials are buried under cleavable surfaces of these crystals14,17 because the kagome planes usually exhibit higher surface energies than their vicinal planes do10, which is a roadblock for direct observation and manipulation of KESs in these materials. This issue, however, can be addressed if we are able to build KESs on a surface of relatively low surface energy (termed surface kagome electronic states, SKESs). SKESs refer to surface electronic states that their wave functions are spatially distributed following the kagome symmetry, namely in a honeycomb lattice where the nodal points of the lattice are comprised of corner-sharing triangles. Building the SKESs enables direct real-space observation and manipulation of rich kagome physics in the atomic limit by surface characterization techniques, such as scanning tunneling microscopy (STM) and atomic force microscopy (AFM). As a consequence, feasible tunability of the SKESs could also be achieved by tip manipulation or molecular beam epitaxy.
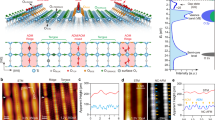
Introduction of a cap** layer composed of nonmetal atoms over a kagome plane often lowers its surface energy10. A SKES is thus built if the cap** layer could inherit the underlying kagome symmetry electronically. Co3Sn2S2, a kagome magnetic Weyl semimetal, is a good candidate to verify whether SKESs could appear on a non-kagome-lattice surface of a kagome crystal. Two major types (Type-I and Type-II) of its surfaces show triangular patterns in STM images10,17,18,19,20, The bulk crystal of Co3Sn2S2 belongs to space group \(R3\bar{m}\), comprising a triangular lattice with constants a = 5.37 Å and c = 13.15 Å11. As shown in Fig. 1a and Supplementary Fig. 1, Co3Sn2S2 has a layered structure composed of a kagome Co3Sn plane (red atoms) sandwiched between two triangular S planes (yellow atoms), which are then further encapsulated by two separate triangular Sn planes (blue atoms). Unlike STM images, which provide information on delocalized electronic states, qPlus nc-AFM images could reveal the spatial gradients of short-range repulsive interactions resulting from localized electronic states at the single-chemical-bond level22,23,24,25,26,27,28,29,30,31. Consequently, it can trace electronic orbitals and/or interactions with unprecedented resolution22,32. Therefore, we performed nc-AFM imaging and short-range force spectroscopy on cleaved Co3Sn2S2 surfaces. The AFM was equipped with a qPlus sensor, and a CO-functionalized tip was used for imaging (Fig. 1b). a Side view of the atomic model of the vertically stacked Sn-S-Co3Sn-S-Sn layers in Co3Sn2S2. b Schematic of the nc-AFM measurements using a qPlus sensor with a CO-functionalized tip. c STM image of the S surface of Co3Sn2S2. d Chemical-bond-resolved nc-AFM image of the S surface taken in the blue square in (c). e Zoomed-in image from (d) to show the incomplete kagome pattern. Three distinct regions within a unit cell with bright, blurry, and dark contrast, which are marked by black solid line triangles, red solid line triangles, and a blue dashed line hexagon, are labeled as αI, βI, and γI regions. The atomic structure of S surface with the underlying Co3Sn plane is superimposed. f STM image of the Type-II surface of Co3Sn2S2. g Chemical-bond-resolved nc-AFM image of the Type-II surface taken in the area marked by a blue square in (f). h Zoomed-in image from (g), showing the kagome pattern. Three distinct regions within a unit cell with bright, blurry, and dark contrast, which are marked by black solid line triangles, red solid line triangles, and a blue dashed line hexagon, are labeled as αII, βII, and γII regions. The atomic structure superimposed is the Sn surface with the underlying S and Co3Sn plane. Scanning parameters: c, f, Vs = −400 mV, It = 100 pA; d, e, amplitude = 100 pm, scanning height = 180 pm lower from a tunneling junction of Vs = −4 mV, It = 10 pA; g, h, amplitude = 100 pm, scanning height = 220 pm lower from a tunneling junction of Vs = −400 mV, It = 100 pA. There are two commonly obtained cleaved Co3Sn2S2 surfaces: one consisting of a few vacancies (Type-I) and the other containing many adatoms (Type-II). By local contact potential difference (LCPD) measurement, the Type-I surface, with a much higher work function, is demonstrated most likely to be the S surface21. However, the Co3Sn and Sn surfaces have comparable surface work functions and cannot be convincingly distinguished by LCPD, thus leaving the Type-II surface identification a remaining issue. Figure 1c shows an STM image of the S (Type-I) surface, which features a triangular lattice with a few S vacancies. In the nc-AFM image (Fig. 1d), the surface S atoms appeared as bright spots with slightly anisotropy (region αI), maintaining their triangular pattern. A further zoomed-in image shown in Fig. 1e shows three line-like features, equivalently distributed in terms of the rotational angle, extending from each S atom. These “lines” converge to rather blurry regions (the βI region) in the image, while the remaining dark region is denoted as γI, implying the formation of i-SKES. a Experimental vertical short-range force spectra measured at the center of the αI (black), βI (red), and γI (blue) regions. b and c DFT optimized surface structure and vertical short-range force spectra on the S surface. d Experimental vertical short-range force spectra measured at the center of the αII (black), βII (red), and γII (blue) regions. e and f DFT optimized surface structure and vertical short-range force spectra on the Sn surface. Spectra displayed in (a) were obtained with an amplitude of 25 pm and 50 pm for that shown in (d). Both experimental vertical short-range force curves were deconvoluted from associated frequency–shift curves using the Sader–Jarvis method45. The Type-II surface, which displays a triangular lattice network decorated with a few adatoms in the STM image (Fig. 1f), shows bright dots assembling in the same triangular lattice in the nc-AFM images acquired at a relatively large tip-sample distance (Supplementary Fig. 2a). In a zoomed-in nc-AFM image, we found the dots are circular in shape (region αII marked in Supplementary Fig. 2b), which outline a blurry triangular (βII), and an indistinctly hexagonal region (γII). As the tip approaches the sample surface (Supplementary Fig. 2c, d), the circular shape of the dots evolves into explicitly triangular in the αII region and the blurry triangles become sharper in region βII. They eventually connect to form a 2D breathing kagome pattern (Fig. 1g). In a zoomed-in image (Fig. 1h), the αII region appears as bright triangles with each side 2.77 Å in length, while the βII region appears bigger but as slightly blurry triangles with each side 3.02 Å in length, which is 9% longer than that of the αII region. The kagome-lattice Type-II surface appears to resemble the structure of the Co3Sn layer, which is, however, less stable compared to the Sn layer under exposure to the surface10. We thus meet difficulties in identifying the Type-II surface as either candidates cannot fit both the kagome-appearance and lower-energy criteria. However, if the energetically preferred Sn-terminated surface could display an electronic kagome-appeared pattern, this issue could be resolved by assigning the Sn surface to the Type-II surface. To verify this assumption, we performed force spectra measurements and DFT calculations. We first measured vertical short-range force spectra on the less controversy S (Type-I) surface to verify the reliability of our experiment and theory comparison. As shown in Fig. 2a, the force spectra at the αI, βI, and γI regions all hit the attractive-repulsive tuning point below 3 Å, with the respective height of the turning point at the αI (γI) regions 0.9 Å higher (0.6 Å lower) than that at the βI region. In Fig. 2b, we tentatively assessed those three regions to the top-layer S (black dashed triangle), the Co3 trimer (red dashed triangle) and the Sn atom (blue dashed hexagon) in the Co3Sn layer, respectively. Given that, our DFT calculations well reproduced those experimental force curves in Fig. 2c, which were plotted using the same color code and exhibit comparable respective heights of the turning positions, i.e., 0.9 Å versus 1.0 Å (0.6 Å versus 0.6 Å) for the αI (γI) region, compellingly supporting those tentative assignments. More importantly, these experiment–theory coincidences, in turn, confirm the assessment of the Type-I surface to the S surface and validate our force spectra comparison method of identifying surface terminations of Co3Sn2S2. Figure 2d plots the experimental force spectra on the Type-II surface, in the same color and spatial schemes used on the Type-I surface. As the tip approaching the surface, the αII region (black) appears the least attractive among all the three regions and first reaches the turning point at 1.92 Å, while that value is 1.60 Å and 1.31 Å for regions βII, and γII, respectively, in which region γII shows a larger attractive force. We modeled the acquiring process of those force curves on both the Co3Sn and Sn surfaces using DFT calculations. Our primary focus is on the relative positions of the turning points of the three regions, which remain qualitatively unchanged by different tips29. For the Co3Sn surface, the surface Sn atoms show appreciable surface relaxations on the Co3Sn surface and are 0.42 Å lifted from the Co3Sn plane (the cross section in Supplementary Fig. 3a). Consequently, the force spectrum on the Sn atom (blue curve in Supplementary Fig. 3b) first reaches its minimum and shows strong repulsion at shorter distances. The two triangular regions, i.e., the surface Co3 trimer sit on a Sn atom and a S atom underneath (Co3/Sn and Co3/S) are less repulsive in comparison with the surface Sn (hexagonal) region, apparently inconsistent with the experimental spectra on the Type-II surface where the two triangular regions exhibit stronger repulsion. This contradiction does not support the assessment of the Co3Sn surface to the Type-II surface. The Sn surface contains triangularly distributed Sn atoms (blue balls in Fig. 2e) in the topmost layer while the same lattice of S atoms (yellow balls), with a lateral shift of (1/3, 1/3) unit cell, is placed in a layer just 0.56 Å below. Each Sn or S atom sits over a Co3 trimer, which could be regarded as a region of triangular symmetry, while the Sn atom in the Co3Sn layer (Sn@Co3Sn) appears a hexagonal symmetry. Thus, we could tentatively assign the Sn and S triangles to triangular regions αII (black) and βII (red), respectively, and the Sn@Co3Sn hexagon to hexagonal region γII (blue). Associated theoretical force spectra (Fig. 2f) show consistent results with the experimental ones in terms of the order of repulsion among those three regions. This verifies our assignment that region αII (βII and γII) represents the Sn (S and Sn@Co3Sn) site, as denoted by the superimposed atomic structure in Fig. 1h. Thereby, based on site-dependent force spectra measurements and corresponding DFT calculations, we identified that Type-II surface, which hosts SKESs, is the Sn surface of Co3Sn2S2. The kagome pattern that appeared on the triangular Sn surface is mostly likely to originate from the Co d orbitals, or their hybridized states with surface atoms. Although the local density of states of the Co d orbitals are much larger than that of the Sn/S p orbitals, the d orbitals are highly localized in real space and electronically screened by the p orbitals of the surface Sn/S atoms. As the repulsive interactions are highly short-ranged in our measurements, the d orbitals in the subsurface layer should play a rather minor role to the tip-recorded repulsive interactions. Therefore, the detected SKESs should be ascribed to the hybridized states of surface Sn/S atoms rather than directly from the underlying Co d orbitals. To elucidate the origin of the kagome-shaped feature on the Sn surface revealed in the nc-AFM images in Fig. 1g and h, the projected local density of states (PLDOS) for the Sn-terminated Co3Sn2S2 surface is plotted in Fig. 3a. It shows a group of electronic states around the Fermi level (EF), which is nearly isolated from other states and ranges from −0.38 to 0.50 eV, as highlighted by the parallel black dashed lines. These states do not consist of the s-orbital component of the surface Sn or S atoms (see Supplementary Fig. 5 for details). We replotted PLDOS between −0.38 and 0.50 eV of the p orbitals of surface Sn and subsurface S atoms and of the d orbitals of the Co3 trimers underneath in Fig. 3b, which indicate strong electronic hybridization between the surface Sn (S) p orbitals, denoted in blue (green), and the d states of the underlying Co3 trimers (in orange). The positions of the four pronounced hybridization peaks are highlighted using four dashed lines. a PLDOS of the Sn surface. Two black dashed lines at −0.38 and 0.50 eV indicate the boundaries of a group of isolated p–d hybridized states. b PLDOS of the top three atomic layers on the Sn surface, from −0.38 to 0.50 eV. Blue and red dashed lines denote the vertically p–d hybridized states between the surface (subsurface) Sn (S) atoms and the Co3 trimers underneath, while the red dashed lines also indicate in-plane (laterally) p–p hybridized states between surface Sn and subsurface S atoms. c Isosurface contour of |ψ| 2, integrated from −0.38 eV to the EF, on the Sn surface superimposed with the atomic structure of the three topmost atomic layers of the Sn surface in the right part. Red solid lines highlight the kagome pattern. d–f Duplicate PLDOS and |ψ| 2 isosurface contour plots, in the same schemes used in (a–c), for the S surface. Boundaries of the isolated p–d hybridized states is at 0.12 and 1.16 eV, as marked by the two black dashed lines in (d). e Zoomed-in PLDOS plotted from 0.12 to 1.16 eV. f Isosurface contour of |ψ| 2 integrated from 0.12 to 1.16 eV. The black solid line triangles mark the hybridized states on S atoms; while the red solid line triangles mark the missing sublattice of the i-SKES. The p–d hybridized states effectively impose the triangular-shaped electron density of the triangular Co3 trimers on those of the surface Sn and S atoms. Figure 3c depicts the square of the wavefunction norms (|ψ | 2) of the occupied hybridized states, showing a triangular-shaped contour at each Sn or S site. Six of these triangles surround a hexagonal area at the Sn@Co3Sn site, where the contour density is the lowest, forming a kagome network on the surface. The plot of the |ψ | 2 contour also reveals the lateral interconnections of the triangularly shaped density contours, indicating their lateral electronic hybridization in real space. The PLDOS confirm this lateral hybridization between Sn and S, which forms electronic states at −0.25 and 0.37 eV (red dashed lines in Fig. 3b). Therefore, through vertical p–d hybridization and subsequent lateral p–p hybridization, the kagome symmetry of the Co3Sn plane underneath is electronically imprinted on the Sn-terminated surface, and an SKES is successfully demonstrated. The hybridization of Sn and S with the Co kagome-lattice network also transfers the nontrivial properties of the kagome electronic states onto the Sn surface. A scanning tunneling spectral (STS) measurement of the Sn surface (Supplementary Fig. 6a) exhibits a sharp peak centered at approximately −6 meV (Pk), as indicated by the red arrow, which fits well with the flat band near EF in the theoretical band structure of the Sn surface (Supplementary Fig. 6b). The band structures projected on surface p orbitals for Sn surface exhibit one flat band and its crossing with a quadratic band at the Г point, as illustrated in Supplementary Fig. 6c, demonstrating that the SKESs have the characteristic feature of the kagome band structures. The position of Pk shifts toward EF under an applied magnetic field perpendicular to the surface with both up- and down-field orientations (Supplementary Fig. 6d). Such an unconventional splitting trend indicates negative flat band magnetism, further confirming the topological origin of the SKESs. DFT calculation results for the S surface are displayed in Fig. 3d–f for comparison with those of the Sn surface. The associated PLDOS spectra were plotted in Fig. 3d. It exhibits a group of isolated states between 0.12 and 1.16 eV, where only the p orbitals of S (both at surface and in bulk) and d orbitals of Co are involved. In this energy window, a zoomed-in PLDOS plot of the surface S and subsurface Co3Sn layers, also indicates vertical hybridization between the surface S p states and the underlayer Co d states, denoted by the dashed lines in Fig. 3e. These vertical p–d hybridizations impose triangular-shaped Co3 electronic states on the surface S atoms, as revealed by the |ψ | 2 contour in Fig. 3f, which could be regarded as an i-SKES because there are no electronic states on top of the other sublattice over the Co kagome network (marked by red solid line triangles in Fig. 3f). As a result, the flat bands observed around EF on the Sn surface were absent on the S surface, even in a wider energy window10,21. a Schematic of SKES (the left part) and i-SKES (the right part) formation through vertical p–d hybridization. b Schematic of the surface planes of sublattice a (blue, SLa plane) and sublattice b (yellow, SLb plane) located on top of the kagome plane (red). c–e Plots of |ψ | 2 isosurface contours of hybridized states. c Contour of a p–d hybridized SKES for SLa = Sn and SLb = Se. Deposition of Ge and Pb on the S surface also generates the same type of SKESs. d Contour of a p–d hybridized asymmetric SKES for SLa = Sb and SLb = S, where the S and Sb sites show substantially different intensities. Deposition of Bi on the S surface also generates the same type of SKESs. e Contour for SLa = Al and SLb = S, where both s–d and p–d mixed hybridizations are involved. The same type of SKESs can be constructed with deposition of Ga, In and Si on S surface, or substitution of SLb elements with Te. We have demonstrated the feasibility of SKES construction on the Sn surface of Co3Sn2S2 by nc-AFM measurements and DFT calculations. The construction strategy requires that (i) the surface and subsurface (if any) atoms fit in a honeycomb lattice, (ii) their in-plane states vertically hybridize with both of the corner-sharing triangular-shaped sublattices of the kagome lattice underneath, and (iii) their hybridized states then subsequently hybridize laterally with each other to form an SKES, as illustrated in the left part of Fig. 4a. In Co3Sn2S2, the Sn-terminated surface approximately meets all these requirements, in which the surface Sn atoms are located on one sublattice, and subsurface S atoms, only 0.56 Å below the surface, reside on the other sublattice. On the other hand, the S-terminated surface in Co3Sn2S2 does not meet requirement (i), thus can only form an i-SKES (see the right part of Fig. 4a). But the additional deposition of hetero- or homo-atoms onto the other sublattice (sublattice plane a, SLa, see Fig. 4b) fills the incomplete part of the i-SKES, enabling the construction of SKESs on the S surface with tunable properties. Furthermore, we could obtain more diverse types of SKESs by substituting the S atoms with Se or Te (sublattice plane b, SLb, see Fig. 4b) during bulk crystal growth. DFT calculations were performed to examine the geometric and electronic structures of the surfaces with modified SLa and SLb planes. Diverse surface electronic hybridizations and SKESs are verified. Similar vertical p–d electronic hybridizations are preserved when SLa = Ge, Sn, or Pb and SLb = S or Se. A typical |ψ | 2 contour of the isolated states near EF for SLa = Sn and SLb = Se is shown in Fig. 4c, revealing kagome features that are comparable to those of the Sn-terminated Co3Sn2S2 surface. The PLDOS (Supplementary Fig. 7a) indicates that the hybridization states comprising Sn and Se p orbitals and d orbitals of Co are restricted within the energy range of −0.5 to 0.5 eV. They are isolated from other electronic states and can therefore be considered pure p–d hybridized SKES near EF. We also constructed a more typical breathing SKES by significantly unbalancing the hybridized states distributed on the SLa and SLb sites, as achieved by substituting the SLa site with heavier elements, like Sb and Bi (Fig. 4d and Supplementary Fig. 7b). For SLa = Al, Ga, In, or Si or SLb = Te, their s orbitals, in addition to the p–d hybridization, are involved in vertical hybridization with Co around EF (Fig. 4e and Supplementary Fig. 7c). The involvement of the s orbital results in the |ψ | 2 contour of the hybridized states appearing spherical in shape, which significantly weakens the kagome feature on the surface. The energetically isolated hybridized states are mixed with other states for surfaces where SLa = P or As, in which the surface kagome feature is eliminated (Supplementary Fig. 7d). These results reveal that the S surface of Co3Sn2S2 can be used as a template for constructing various SKESs with tailored properties. In summary, we have demonstrated a strategy for constructing energetically stable SKESs that involves electronically imprinting the kagome-lattice symmetry of an undersurface layer through vertical p–d and lateral p–p electronic hybridizations. The strategy was verified using the Sn surface of Co3Sn2S2, which is a prototypical kagome magnetic Weyl semimetal. By combining chemical-bond-resolved nc-AFM images, vertical short-range force spectra, and DFT calculations, we explicitly found SKESs on the Sn surface of Co3Sn2S2. Our DFT calculations revealed that these states originate from the strong vertical electronic hybridization between triangularly arranged surface Sn or subsurface S atoms and the Co3 trimers of the Co3Sn kagome lattice underneath, accompanied by lateral hybridizations of the surface Sn and subsurface S states. STS measurements verified the appearance of kagome symmetry featured topological properties on the Sn surface. To generalize this strategy, DFT calculations explored the surface electronic structure variations for atomic substitutions of surface Sn (subsurface S) with III-A, V-A, and IV-A (Se or Te) elements. We theoretically demonstrated the feasibility of constructing surface kagome structures by depositing group III-A or IV-A elements on the S surface. We also noticed some recent reports on other kagome materials, such as AV3Sb5 and RV6Sn6, show the typical kagome bands on honeycomb lattice surfaces14,33,34,35,36, which coincide with the proposed mechanism in this work. The demonstration of nc-AFM for investigating localized electronic surface states aside, our strategy for building SKESs enriches the design routes to construct semi-metallic 2D kagome materials with topologically nontrivial properties. A Sn/Pb mixed flux was used to grow single crystals of Co3Sn2S2. First, the starting materials were mixed at a molar ratio of Co:S:Sn:Pb = 12:8:35:45 (Co (99.95% Alfa), Sn (99.999% Alfa), S (99.999% Alfa), and Pb (99.999% Alfa)). The mixture was placed in an Al2O3 crucible sealed in a quartz tube that was slowly heated to 673 K for 6 h and then maintained for 6 h to avoid heavy loss of sulfur. Thereafter, the quartz tube was heated to 1323 K for 6 h and maintained for 6 h before slowly cooling to 973 K over 70 h. At 973 K, rapid decanting and subsequent spinning in a centrifuge were performed to remove flux. Finally, hexagonal-plate single crystals with diameters of 2–5 mm were obtained. Energy-dispersive X-ray spectroscopy and X-ray diffraction were used to determine the compositions and phase structures of the crystals. Non-contact AFM measurements were performed on a combined nc-AFM/STM system (Createc) at LHe temperature with a base pressure lower than 2 × 10−10 mbar. All measurements were performed using a commercial qPlus tuning fork sensor37 in the frequency modulation mode with a Pt/Ir tip at 4.5 K. The resonance frequency of the AFM tuning fork was 27.9 kHz, and the stiffness was approximately 1800 N/m. The Co3Sn2S2 samples were cleaved at <10 K in an ultrahigh vacuum chamber and transferred to the nc-AFM/STM head within 10 s. nc-AFM images and short-range force spectra were recorded using CO-functionalized tips. The oscillation amplitudes used in all measurements are stated in the corresponding figure captions. DFT calculations were performed using the generalized gradient approximation for the exchange-correlation potential, the projector augmented wave method38 and a plane-wave basis set as implemented in the Vienna ab-initio simulation package39,40. The energy cutoff for the plane-wave basis was set to 400 eV for structural relaxations and 500 eV for the energy and electronic structure calculations. Two k-meshes of 7 × 7 × 1 and 11 × 11 × 1 were adopted for the structural relaxations and total energy (electronic structure) calculations, respectively. The mesh density of the k points was fixed when performing the related calculations with primitive cells. In geometric structure relaxation, van der Waals (vdW) interactions were considered at the vdW-DF level with the optB86b functional as the exchange functional (optB86b-vdW)41,42. Symmetrical slab models were employed, and the surface atoms were fully relaxed until the residual force per atom was less than 0.005 eV Å-1. To avoid image interactions between adjacent unit cells, a vacuum layer of more than 20 Å thick was added to the slab cell perpendicular to the surface. The optimized lattice constants of bulk Co3Sn2S2 are 5.37 and 13.15 Å along the a and c directions, respectively. A tip-sample model was established for the force spectral calculations. In particular, the AFM tip was modeled using a five-layer thick Pt(111) cluster, the bottom of which adsorbs a CO molecule, and a (4 × 4) Co3Sn2S2 slab was employed to model the sample surface. Our tip model exhibited a significant p-wave feature, which contributes to the high resolution in images acquired using CO-functionalized tips43,44. Energetically favorable magnetic ground states were calculated and used for all the tip-sample configurations.Results
SKESs on Type-II surface of Co3Sn2S2
Identification of Type-II surface
p–d hybridization between surface atoms and the kagome plane
Strategy for constructing a family of SKESs
Discussion
Methods
Single-crystal growth of Co3Sn2S2
QPlus nc-AFM measurements
DFT calculations
Data availability
All data that support the findings of this study are present in the paper and the Supplementary Information. Further information can be acquired from the corresponding authors upon reasonable request.
References
Bergman, D. L., Wu, C. & Balents, L. Band touching from real-space topology in frustrated hop** models. Phys. Rev. B 78, 125104 (2008).
Guo, H. M. & Franz, M. Topological insulator on the kagome lattice. Phys. Rev. B 80, 113102 (2009).
Mazin, I. I. et al. Theoretical prediction of a strongly correlated Dirac metal. Nat. Commun. 5, 4261 (2014).
Nakatsuji, S., Kiyohara, N. & Higo, T. Large anomalous Hall effect in a non-collinear antiferromagnet at room temperature. Nature 527, 212–215 (2015).
Kuroda, K. et al. Evidence for magnetic Weyl fermions in a correlated metal. Nat. Mater. 16, 1090–1095 (2017).
Ye, L. et al. Massive Dirac fermions in a ferromagnetic kagome metal. Nature 555, 638–642 (2018).
Lin, Z. et al. Flatbands and emergent ferromagnetic ordering in Fe3Sn2 kagome lattices. Phys. Rev. Lett. 121, 096401 (2018).
Wang, Q. et al. Large intrinsic anomalous Hall effect in half-metallic ferromagnet Co3Sn2S2 with magnetic Weyl fermions. Nat. Commun. 9, 3681 (2018).
Liu, E. et al. Giant anomalous Hall effect in a ferromagnetic kagome-lattice semimetal. Nat. Phys. 14, 1125–1131 (2018).
Morali, N. et al. Fermi-arc diversity on surface terminations of the magnetic Weyl semimetal Co3Sn2S2. Science 365, 1286–1291 (2019).
Liu, D. F. et al. Magnetic Weyl semimetal phase in a kagome crystal. Science 365, 1282–1285 (2019).
Kang, M. et al. Dirac fermions and flat bands in the ideal kagome metal FeSn. Nat. Mater. 19, 163–169 (2020).
Yin, J.-X. et al. Quantum-limit Chern topological magnetism in TbMn6Sn6. Nature 583, 533–536 (2020).
Chen, H. et al. Roton pair density wave in a strong-coupling kagome superconductor. Nature 599, 222–228 (2021).
Neupert, T., Denner, M. M., Yin, J.-X., Thomale, R. & Hasan, M. Z. Charge order and superconductivity in kagome materials. Nat. Phys. 18, 137–143 (2022).
Hutter, A., Wootton, J. R. & Loss, D. Parafermions in a kagome lattice of qubits for topological quantum computation. Phys. Rev. X 5, 041040 (2015).
Yin, J.-X. et al. Negative flat band magnetism in a spin–orbit-coupled correlated kagome magnet. Nat. Phys. 15, 443–448 (2019).
Jiao, L. et al. Signatures for half-metallicity and nontrivial surface states in the kagome lattice Weyl semimetal Co3Sn2S2. Phys. Rev. B 99, 245158 (2019).
Howard, S. et al. Observation of linearly dispersive edge modes in a magnetic Weyl semimetal Co3Sn2S2. Preprint at https://arxiv.org/abs/1910.11205 (2019).
Yin, J.-X. et al. Spin-orbit quantum impurity in a topological magnet. Nat. Commun. 11, 4415 (2020).
**ng, Y. et al. Localized spin-orbit polaron in magnetic Weyl semimetal Co3Sn2S2. Nat. Commun. 11, 5613 (2020).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Emmrich, M. et al. Subatomic resolution force microscopy reveals internal structure and adsorption sites of small iron clusters. Science 348, 308–311 (2015).
Riss, A. et al. Imaging single-molecule reaction intermediates stabilized by surface dissipation and entropy. Nat. Chem. 8, 678–683 (2016).
Kawai, S. et al. Superlubricity of graphene nanoribbons on gold surfaces. Science 351, 957–961 (2016).
Pavliček, N. et al. Synthesis and characterization of triangulene. Nat. Nano 12, 308–311 (2017).
Kaiser, K. et al. An sp-hybridized molecular carbon allotrope, cyclo 18 carbon. Science 365, 1299–1301 (2019).
Berwanger, J., Polesya, S., Mankovsky, S., Ebert, H. & Giessibl, F. J. Atomically resolved chemical reactivity of small Fe clusters. Phys. Rev. Lett. 124, 096001 (2020).
Qi, J. et al. Force-activated isomerization of a single molecule. J. Am. Chem. Soc. 142, 10673–10680 (2020).
Huber, F. et al. Chemical bond formation showing a transition from physisorption to chemisorption. Science 366, 235–238 (2019).
Liebig, A., Hapala, P., Weymouth, A. J. & Giessibl, F. J. Quantifying the evolution of atomic interaction of a complex surface with a functionalized atomic force microscopy tip. Sci. Rep. 10, 14104 (2020).
Zhao, H. et al. Cascade of correlated electron states in the kagome superconductor CsV3Sb5. Nature 599, 216–221 (2021).
Peng, S. et al. Realizing kagome band structure in two-dimensional kagome surface states of RV6Sn6 (R=Gd, Ho). Phys. Rev. Lett. 127, 266401 (2021).
Tan, H. & Yan, B. Abundant lattice instability in kagome metal ScV6Sn6. Phys. Rev. Lett. 130, 266402 (2023).
Cheng, S. et al. Nanoscale visualization and spectral fingerprints of the charge order in ScV6Sn6 distinct from other kagome metals. Preprint at https://arxiv.org/abs/2302.12227 (2023).
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956–3958 (1998).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Klimeš, J., Bowler, D. R. & Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 83, 195131 (2011).
Klimeš, J., Bowler, D. R. & Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Phys. Condens. Matter 22, 022201 (2009).
Gross, L. et al. High-resolution molecular orbital imaging using a p-Wave STM tip. Phys. Rev. Lett. 107, 086101 (2011).
Mönig, H. et al. Quantitative assessment of intermolecular interactions by atomic force microscopy imaging using copper oxide tips. Nat. Nanotech. 13, 371–375 (2018).
Sader, J. E. & Jarvis, S. P. Accurate formulas for interaction force and energy in frequency modulation force spectroscopy. Appl. Phys. Lett. 84, 1801–1803 (2004).
Acknowledgements
The work is supported by grants from the National Natural Science Foundation of China (61888102 (H.-J.G.), 61761166009 (W.J.), 11974422 (W.J.), and 12104313 (X.K.)), the National Key Research and Development Projects of China (2019YFA0308500 (H.-J.G. and L.H.), 2018YFE0202700 (W.J.), 2022YFA1204100 (H.Y. and H.Chen)and 2018YFA0305800 (L.H., Y.Z., and X.L.)), the Chinese Academy of Sciences (XDB30000000 (W.J., L.H. and H.Chen), YSBR-003 (L.H., H.Chen and X.L.)), and the Innovation Program of Quantum Science and Technology (2021ZD0302700 (H.-J.G., H.Y., L.H and H.Chen)). W.J. gratefully acknowledges the Fundamental Research Funds for the Central Universities of China and the Research Funds of Renmin University of China (22XNKJ30). X.K. gratefully acknowledges funding by the Department of Science and Technology of Guangdong Province grant 2021QN02L820 and the Shenzhen Natural Science Fund (the Stable Support Plan Program 20220810161616001). Z.W. is supported by the US DOE, Basic Energy Sciences Grant No. DE-FG02-99ER45747. X.K. thanks Prof. Hong Guo for his financial support to the work done at McGill University.
Author information
Authors and Affiliations
Contributions
H.-J.G. and W.J. conceive of this project; L.H., Q.Z., Y.X., H.Chen, Z.C. and X.L. performed nc-AFM/STM experiments with the guidance of H.-J.G.; X.K., Z.H., Y.Z., H.Cheng and W.J. carried out theoretical calculations and analysis; H.Y., X.Q., E.L. and H.L. prepared the samples; Y.L., S.Z. and J.Q. helped in plotting the figures; L.H., Q.Z., X.K., Z.W., W.J. and H.-J.G. write the manuscript with inputs from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shaowei Li and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, L., Kong, X., Zheng, Q. et al. Discovery and construction of surface kagome electronic states induced by p-d electronic hybridization in Co3Sn2S2. Nat Commun 14, 5230 (2023). https://doi.org/10.1038/s41467-023-40942-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40942-2
- Springer Nature Limited
This article is cited by
-
Kagomerization of transition metal monolayers induced by two-dimensional hexagonal boron nitride
Nature Communications (2024)