Abstract
The development of precious-metal-free catalysts to promote the sustainable production of fuels and chemicals from biomass remains an important and challenging target. Here, we report the efficient hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to 2,5-dimethylfuran over a unique core-shell structured catalyst, Co@CoO that affords the highest productivity among all catalysts, including noble-metal-based catalysts, reported to date. Surprisingly, we find that the catalytically active sites reside on the shell of CoO with oxygen vacancies rather than the metallic Co. The combination of various spectroscopic experiments and computational modelling reveals that the CoO shell incorporating oxygen vacancies not only drives the heterolytic cleavage, but also the homolytic cleavage of H2 to yield more active Hδ− species, resulting in the exceptional catalytic activity. Co@CoO also exhibits excellent activity toward the direct hydrodeoxygenation of lignin model compounds. This study unlocks, for the first time, the potential of simple metal-oxide-based catalysts for the hydrodeoxygenation of renewable biomass to chemical feedstocks.
Similar content being viewed by others
Introduction
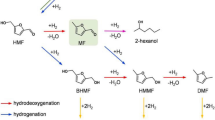
Biomass is the only renewable resource of organic carbons in nature and their conversion to value-added chemicals and liquid fuels is of vital importance in achieving global carbon neutralisation1,2. Cellulose-derived 5-hydroxymethylfurfural (HMF) is widely recognised as a platform chemical for the synthesis of sustainable liquid fuels and chemicals3,4. Particularly, the selective hydrogenolysis of HMF to 2,5-dimethylfuran (DMF) as biofuels and feedstocks of renewable p-xylene has attracted much interest5,6. A great deal of effort has been devoted to develo** supported metal catalysts for this reaction, and state-of-the-art catalysts are based upon Ru, Pd, Pt, Ni and Cu materials7,8,9,10,11. We have designed cobalt oxide-supported ruthenium (Ru/Co3O4) and cobalt/nickel [(Co)Ni/Co3O4] catalysts that show DMF yields of 93% and 70–76%, respectively, at 130 °C for 24 h7,8. Schüth et al. developed a hollow platinum-cobalt bimetallic nanoparticle (PtCo@HCS) catalyst, which achieved a high yield of DMF (98%) at 180 °C for 2 h10. Esteves et al. investigated various supported copper catalysts and identified a high yield of DMF (93%) over Cu/Fe2O3-Al2O3 at 150 °C for 10 h11. To date, metal and harsh reaction condition (i.e., high temperature and/or long reaction time) are almost indispensable to achieve the high yield of DMF. It is widely accepted that the homolytic dissociation of H2 occurs on these metal catalysts, generating free radicals (H·) to drive the subsequent hydrogenolysis12. Recently, it is reported that Hδ− species obtained via heterolytic dissociation of H2 showed enhanced catalytic performance13,14,15,16,17. Thus, the development of new catalysts that can generate Hδ− species hold great promise to promote the hydrogenolysis of HMF under mild reaction conditions.
Although single-atom catalysts can catalyse the heterolytic cleavage of H213,14,15, there is complicity associated with their preparation and thermodynamic stability. Meanwhile, metal oxides with a high concentration of surface defects are reported as emerging catalysts with high activity for the heterolytic cleavage of H216,17,18,19,20,21,Density functional theory studies In this work, all spin-polarized DFT calculations were carried out using the Vienna Ab-initio Simulation Package (VASP)53. The projector augmented wave (PAW) method54 and the Perdew−Burke−Ernzerhof (PBE)55 functional under the generalized gradient approximation (GGA)56 were applied throughout the calculations. The kinetic energy cut-off was set to 400 eV, and the force threshold in structure optimization was 0.05 eV/Å. We used a large vacuum gap of 15 Å to eliminate the interactions between neighbouring slabs. By adopting these calculation settings, the optimized lattice constant of CoO (P1) is 4.248 Å, which is in good agreement with the experimental value of 4.267 Å57. The transition states (TS) of surface reactions were located using a constrained optimization scheme and were verified when (i) all forces on the relaxed atoms vanish and (ii) the total energy is a maximum along the reaction coordination but it is a minimum with respect to the rest of the degrees of freedom58,59,60. The adsorption energy of species X on the surface (Eads(X)) was calculated with where EX/slab is the calculated total energy of the adsorption system, while Eslab and EX are calculated energies of the clean surface and the gas-phase molecule X, respectively. Obviously, a positive value of Eads(X) indicates an exothermic adsorption process, and the more positive the Eads(X) is, the more strongly the adsorbate X binds to the surface. The oxygen vacancy formation energy (EOV) was calculated according to where Eslab-OV is the total energy of the surface with one oxygen vacancy, and EO2 is the energy of a gas-phase O2 molecule. For the model construction, we built a p(2 × 3) surface slab containing five atomic layers for the CoO(100) surface (a = 12.74 Å; b = 8.67 Å; c = 23.50 Å; α = β = γ = 90°), and the top four CoO layers were allowed to relax, while the bottom atomic layer was kept fixed to mimic the bulk region. A 2 × 2 × 1 k-point mesh was used in calculations of all these models. Note that the on-site Coulomb interaction correction is necessary for the appropriate description of the Co 3d electrons, and all calculations are performed with U = 5.1 eV and J = 1.0 eV, which are consistent with the values determined by previous studies61,62. In addition, we tested the effect of the spin state of 3d electrons in Co2+ in the optimization of CoO, and found that the high‐spin antiferromagnetic arrangement was the most stable state, and the calculated magnetic moment of 2.74 µB obtained from the difference in spin-up and spin-down densities is consistent with literature reports63,64,65.
Data availability
The data supporting the findings of this study are available within the article, or available from the authors upon reasonable request.
References
Zhang, Z., Song, J. & Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 117, 6834–6880 (2017).
Sun, Z., Fridrich, B., de Santi, A., Elangovan, S. & Barta, K. Bright side of lignin depolymerization: toward new platform chemicals. Chem. Rev. 118, 614–678 (2018).
Chen, S., Wojcieszak, R., Dumeignil, F., Marceau, E. & Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 118, 11023–11117 (2018).
Roman-Leshkov, Y., Barrett, C. J., Liu, Z. Y. & Dumesic, J. A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 447, 982–985 (2007).
Maki-Arvela, P., Ruiz, D. & Murzin, D. Y. Catalytic hydrogenation/hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran. ChemSusChem 14, 150–168 (2021).
Dutta, S. & Bhat, N.S., Catalytic synthesis of renewable p-xylene from biomass-derived 2,5-dimethylfuran: a mini review. Biomass Conv. Bioref. https://doi.org/10.1007/s13399-020-01042-z (2020).
Zu, Y. et al. Efficient production of the liquid fuel 2,5-dimethylfuran from 5-hydroxymethylfurfural over Ru/Co3O4 catalyst. Appl. Catal. B: Environ. 146, 244–248 (2014).
Yang, P. et al. Catalytic production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over Ni/Co3O4 catalyst. Catal. Commun. 66, 55–59 (2015).
Thananatthanachon, T. & Rauchfuss, T. B. Efficient production of the liquid fuel 2,5-dimethylfuran from fructose using formic acid as a reagent. Angew. Chem. Int. Ed. 49, 6616–6618 (2010).
Wang, G.-H. et al. Platinum–cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nat. Mater. 13, 293–300 (2014).
Esteves, L. M. et al. Effect of support on selective 5-hydroxymethylfurfural hydrogenation towards 2,5-dimethylfuran over copper catalysts. Fuel 270, 117524 (2020).
Van Lent, R. et al. Site-specific reactivity of molecules with surface defects-the case of H2 dissociation on Pt. Science 363, 155–157 (2019).
Liu, P. X. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–801 (2016).
Ye, T. N. et al. Stable single platinum atoms trapped in sub-nanometer cavities in 12CaO.7Al2O3 for chemoselective hydrogenation of nitroarenes. Nat. Commun. 11, 1020 (2020).
Li, S. et al. Selective hydrogenation of 5-(hydroxymethyl)furfural to 5-methylfurfural over single atomic metals anchored on Nb2O5. Nat. Commun. 12, 584 (2021).
Zhang, Z. et al. Metal-free ceria catalysis for selective hydrogenation of crotonaldehyde. ACS Catal. 10, 14560–14566 (2020).
Zhang, S. et al. Solid frustrated-Lewis-pair catalysts constructed by regulations on surface defects of porous nanorods of CeO2. Nat. Commun. 8, 15266 (2017).
Li, Z. et al. Interaction of hydrogen with ceria: hydroxylation, reduction, and hydride formation on the surface and in the bulk,. Chem. Eur. J. 27, 5268–5276 (2021).
Joubert, J. et al. Heterolytic Splitting of H2 and CH4 on γ-alumina as a structural probe for defect sites. J. Phys. Chem. B 110, 23944–23950 (2006).
Gribov, E. N. et al. Vibrational and thermodynamic properties of H2 adsorbed on MgO in the 300-20 K interval. J. Phys. Chem. B 108, 16174–16186 (2004).
Zhao, Y., Rousseau, R., Li, J. & Mei, D. Theoretical study of syngas hydrogenation to methanol on the polar Zn-terminated ZnO (0001) surface. J. Phys. Chem. C. 116, 15952–15961 (2012).
Wu, Z., **ong, F., Wang, Z. & Huang, W. Thermal-, photo- and electron-induced reactivity of hydrogen species on rutile TiO2(110) surface: role of oxygen vacancy. Chin. Chem. Lett. 29, 752–756 (2018).
Wang, X., Liang, X., Li, J. & Li, Q. Catalytic hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to biofuel 2, 5-dimethylfuran. Appl. Catal. A: Gen. 576, 85–95 (2019).
Wei, Z., Lou, J., Li, Z. & Liu, Y. One-pot production of 2,5-dimethylfuran from fructose over Ru/C and a Lewis–Brønsted acid mixture in N,N-dimethylformamide. Catal. Sci. Technol. 6, 6217–6225 (2016).
Nagpure, A. S. et al. Renewable fuels from biomass-derived compounds: Ru-containing hydrotalcites as catalysts for conversion of HMF to 2,5-dimethylfuran. Catal. Sci. Technol. 5, 1463–1472 (2015).
Gao, Z., Fan, G., Liu, M., Yang, L. & Li, F. Dandelion-like cobalt oxide microsphere-supported RuCo bimetallic catalyst for highly efficient hydrogenolysis of 5-hydroxymethylfurfural. Appl. Catal. B: Environ. 237, 649–659 (2018).
Li, Q. et al. Ruthenium supported on CoFe layered double oxide for selective hydrogenation of 5-hydroxymethylfurfural. Mol. Catal. 431, 32–38 (2017).
Gan, T. et al. Facile synthesis of kilogram-scale Co-alloyed Pt single-atom catalysts via ball milling for hydrodeoxygenation of 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 8, 8692–8699 (2020).
Shi, J., Wang, Y., Yu, X., Du, W. & Hou, Z. Production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over reduced graphene oxides supported Pt catalyst under mild conditions. Fuel 163, 74–79 (2016).
Goyal, R. et al. Studies of synergy between metal–support interfaces and selective hydrogenation of HMF to DMF in water. J. Catal. 340, 248–260 (2016).
Saha, B., Bohn, C. M. & Abu-Omar, M. M. Zinc-assisted hydrodeoxygenation of biomass-derived 5-hydroxymethylfurfural to 2,5-dimethylfuran. ChemSusChem 7, 3095–3101 (2014).
Kong, X. et al. Switchable synthesis of 2,5-dimethylfuran and 2,5-dihydroxymethyltetrahydrofuran from 5-hydroxymethylfurfural over Raney Ni catalyst. RSC Adv. 4, 60467–60472 (2014).
Gyngazova, M. S., Negahdar, L., Blumenthal, L. C. & Palkovits, R. Experimental and kinetic analysis of the liquid phase hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over carbon-supported nickel catalysts. Chem. Eng. Sci. 173, 455–464 (2017).
Chen, M.-Y., Chen, C.-B., Zada, B. & Fu, Y. Perovskite type oxide-supported Ni catalysts for the production of 2,5-dimethylfuran from biomass-derived 5-hydroxymethylfurfural. Green. Chem. 18, 3858–3866 (2016).
Yang, P. P., **a, Q. N., Liu, X. H. & Wang, Y. Q. High-yield production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over carbon supported Ni-Co bimetallic catalyst. J. Energy Chem. 25, 1015–1020 (2016).
Yang, P. P., **a, Q. N., Liu, X. H. & Wang, Y. Q. Catalytic transfer hydrogenation/hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Ni-Co/C catalyst. Fuel 187, 159–166 (2017).
Chen, B. B., Li, F., Huang, Z. & Yuan, G. Carbon-coated Cu-Co bimetallic nanoparticles as selective and recyclable catalysts for production of biofuel 2,5-dimethylfuran. Appl. Catal. B: Environ. 200, 192–199 (2017).
Li, D. et al. Selective hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Co3O4 catalyst by controlled reduction. J. Energy Chem. 30, 34–41 (2019).
**ao, T., Liu, X., Xu, G. & Zhang, Y. Phase tuning of ZrO2 supported cobalt catalysts for hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran under mild conditions. Appl. Catal. B: Environ. 295, 120270 (2021).
Bottari, G. et al. Copper-zinc alloy nanopowder: a robust precious-metal-free catalyst for the conversion of 5-hydroxymethylfurfural. ChemSusChem 8, 1323–1327 (2015).
Srivastava, S., Jadeja, G. C. & Parikh, J. Synergism studies on alumina-supported copper-nickel catalysts towards furfural and 5-hydroxymethylfurfural hydrogenation. J. Mol. Catal. A-Chem. 426, 244–256 (2017).
Seemala, B., Cai, C. M., Wyman, C. E. & Christopher, P. Support induced control of surface composition in Cu–Ni/TiO2 catalysts enables high yield Co-conversion of HMF and furfural to methylated furans. ACS Catal. 7, 4070–4082 (2017).
Luo, J. et al. Mechanisms for high selectivity in the hydrodeoxygenation of 5-hydroxymethylfurfural over PtCo nanocrystals. ACS Catal. 6, 4095–4104 (2016).
Luo, J. et al. Base metal-Pt alloys: a general route to high selectivity and stability in the production of biofuels from HMF. Appl. Catal. B: Environ. 199, 439–446 (2016).
Lukashuk, L. et al. Operando XAS and NAP-XPS studies of preferential CO oxidation on Co3O4 and CeO2-Co3O4 catalysts. J. Catal. 344, 1–15 (2016).
Zhang, L. et al. Atomically dispersed Co catalyst for efficient hydrodeoxygenation of lignin-derived species and hydrogenation of nitroaromatics. ACS Catal. 10, 8672–8682 (2020).
Zhang, S. et al. Restructuring transition metal oxide nanorods for 100% selectivity in reduction of nitric oxide with carbon monoxide. Nano Lett. 13, 3310–3314 (2013).
Chin, R. L. & Hercules, D. M. Surface spectroscopic characterization of cobalt-alumina catalysts. J. Phys. Chem. 86, 360–367 (1982).
Noronha, F. B. et al. Characterization of niobia-supported palladium-cobalt catalysts. J. Phys. Chem. B 104, 5478–5485 (2000).
Ren, J. et al. Activation of formyl C-H and hydroxyl O-H bonds in HMF by the CuO(111) and Co3O4(110) surfaces: a DFT study. Appl. Surf. Sci. 456, 174–183 (2018).
Antonov, V. E. et al. Neutron scattering studies of -CoH. J. Alloy. Compd. 404–406, 73–76 (2005).
Polo-Garzon, F. et al. Elucidation of the reaction mechanism for high-temperature water gas shift over an industrial-type copper-chromium-iron oxide catalyst. J. Am. Chem. Soc. 141, 7990–7999 (2019).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, J. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Teter, M. P., Payne, M. C. & Allan, D. C. Solution of Schrodinger's equation for large systems. Phys. Rev. B 40, 12255–12263 (1989).
Redman, M. J. & Steward, E. G. Cobaltous oxide with the zinc blende/wurtzite-type crystal structure. Nature 193, 867–867 (1962).
Alavi, A., Hu, P., Deutsch, T., Silvestrelli, P. L. & Hutter, J. CO oxidation on Pt(111): An Ab initio density functional theory study. Phys. Rev. Lett. 80, 3650–3653 (1998).
Liu, Z. P. & Hu, P. General rules for predicting where a catalytic reaction should occur on metal surfaces: A density functional theory study of C-H and C-O bond breaking/making on flat, stepped, and kinked metal surfaces. J. Am. Chem. Soc. 125, 1958–1967 (2003).
Michaelides, A. et al. Identification of general linear relationships between activation energies and enthalpy changes for dissociation reactions at surfaces. J. Am. Chem. Soc. 125, 3704–3705 (2003).
Archer, T., Hanafin, R. & Sanvito, S. Magnetism of CoO polymorphs: density functional theory and monte Carlo simulations. Phys. Rev. B 78, 014431 (2008).
Pickett, W. E., Erwin, S. C. & Ethridge, E. C. Reformulation of the LDA+U method for a local-orbital basis. Phys. Rev. B 58, 1201–1209 (1998).
Solovyev, I. V., Liechtenstein, A. I. & Terakura, K. Is Hund's second rule responsible for the orbital magnetism in solids? Phys. Rev. Lett. 80, 5758–5761 (1998).
Rödl, C., Fuchs, F., Furthmüller, J. & Bechstedt, F. Quasiparticle band structures of the antiferromagnetic transition-metal oxides MnO, FeO, CoO, and NiO. Phys. Rev. B 79, 235114 (2009).
Wdowik, U. D. & Parlinski, K. Lattice dynamics of CoO from first principles. Phys. Rev. B 75, 104306 (2007).
Acknowledgements
This project was supported financially by the National Natural Science Foundation of China (No. 21832002, 21825301, 21872050, 21808063, 22002043), Shanghai Municipal Science and Technology Major Project (Grant No.2018SHZDZX03), the Programme of Introducing Talents of Discipline to Universities (B16017), China and EPSRC (EP/V056409), UK. This research used Beamline VISION at the Spallation Neutron Source, a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory.
Author information
Authors and Affiliations
Contributions
S.X., L.D., X.H.L. and Y.G.: preparation and characterization of catalysts, and performing the catalytic reactions. Z.Q.W. and X.Q.G.: DFT calculations. X.H., L.L.D., Y.C., A.J.R.-C. and S.Y.: collection and analysis of neutron scattering data. Q.L. and Y.F.H.: collection and analysis of XAS. S.Y., X.Q.G. and Y.Q.W.: overall direction of the project. S.X., L.D., S.Y. and Y.Q.W. wrote the manuscript with the help from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
**ang, S., Dong, L., Wang, ZQ. et al. A unique Co@CoO catalyst for hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to 2,5-dimethylfuran. Nat Commun 13, 3657 (2022). https://doi.org/10.1038/s41467-022-31362-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-31362-9
- Springer Nature Limited
This article is cited by
-
Homolytic H2 dissociation for enhanced hydrogenation catalysis on oxides
Nature Communications (2024)
-
Engineering the Interface Between Au Nanoparticles and CoO-Ov to Enhance the Catalytic Performance of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF)
Chemical Research in Chinese Universities (2024)
-
Tripodal Pd metallenes mediated by Nb2C MXenes for boosting alkynes semihydrogenation
Nature Communications (2023)
-
Enhancing polyol/sugar cascade oxidation to formic acid with defect rich MnO2 catalysts
Nature Communications (2023)