Abstract
Stem cell transplantation, as a promising treatment for central nervous system (CNS) diseases, has been hampered by crucial issues such as a low cell survival rate, incomplete differentiation, and limited neurite outgrowth in vivo. Addressing these hurdles, scientists have designed bioscaffolds that mimic the natural tissue microenvironment to deliver physical and soluble cues. However, several significant obstacles including burst release of drugs, insufficient cellular adhesion support, and slow scaffold degradation rate remain to be overcome before the full potential of bioscaffold–based stem-cell therapies can be realized. To this end, we developed a biodegradable nanoscaffold-based method for enhanced stem cell transplantation, differentiation, and drug delivery. These findings collectively support the therapeutic potential of our biodegradable hybrid inorganic (BHI) nanoscaffolds for advanced stem cell transplantation and neural tissue engineering.
Similar content being viewed by others
Introduction
Develo** reliable therapeutic methods to treat central nervous system (CNS) diseases (e.g., Alzheimer’s and Parkinson’s diseases), degeneration in the aging brain, and CNS injuries (e.g., spinal cord injury (SCI) and traumatic brain injuries) has been a major challenge due to the complex and dynamic cellular microenvironment during the disease progression1,2. Several current therapeutic approaches have aimed to restore neural signaling, reduce neuroinflammation, and prevent subsequent damage to the injured area using stem cell transplantations3,4,5,6. Given the intrinsically limited regenerative abilities of the CNS and the highly complex inhibitory environment of the damaged tissues, stem cell transplantation has great potential to regenerate a robust population of functional neural cells such as neurons and oligodendrocytes, thereby re-establishing disrupted neural circuits in the damaged CNS areas4,7,8,9,10. However, several pertinent obstacles hinder advances in stem cell transplantation. First, due to the inflammatory nature of the injured regions, many transplanted cells perish soon after transplantation11. Second, the extracellular matrix (ECM) of the damaged areas is not conducive to stem cell survival and differentiation2,12. Therefore, to address the aforementioned issues and facilitate the progress of stem cell therapies, there is a clear need to develop an innovative approach to increase the survival rate of transplanted stem cells and to better control stem cell fate in vivo, which can lead to the recovery of the damaged neural functions and the repair of neuronal connections in a more effective manner.
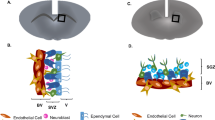
To this end, we report a biodegradable hybrid inorganic (BHI) nanoscaffold-based method to improve the transplantation of human patient-derived neural stem cells (NSCs) and to control the differentiation of transplanted NSCs in a highly selective and efficient way. Further, as a proof-of-concept demonstration, we combined the spatiotemporal delivery of therapeutic molecules with enhanced stem cell survival and differentiation using BHI-nanoscaffold in a mouse model of SCI. Specifically, our developed three-dimensional (3D) BHI-nanoscaffolds (Fig. 1) have unique benefits for advanced stem cell therapies: (i) wide-range tunable biodegradation; (ii) upregulated ECM-protein binding affinity; (iii) highly efficient drug loading with sustained drug delivery capability; and (iv) innovative magnetic resonance imaging (MRI)-based drug release monitoring (Fig. 1a-c). Hybrid biomaterial scaffolds have been demonstrated to mimic the natural microenvironment for stem cell-based tissue engineering13,14,15,16,17,18,19,20,21,22. In this regard, scientists including our group, have recently reported that low-dimensional (0D, 1D, and 2D) inorganic and carbon nanomaterial (e.g., TiO2 nanotubes, carbon nanotubes, and graphene)-based scaffolds, having unique biological and physiochemical properties, and nanotopographies, can effectively control stem cell behaviors in vitro, as well as in vivo23,24,25,26,27,28,29,30,31. However, these inorganic and carbon-based nanoscaffolds are intrinsically limited by their non-biodegradability and restricted biocompatibility, thereby delaying their wide clinical applications. On the contrary, MnO2 nanomaterials have proven to be biodegradable in other bioapplications such as cancer therapies, with MRI active Mn2+ ions as a degradation product32,33,10b to 4 mm:107.5 mm2 in Supplementary Fig. 10c), which was achieved by filtrating same amount of MnO2 nanosheets (1.0 ml of 3.0 mg per ml) but reduced the filtrating area by 10 times; (iii) protein amount in the scaffold (MnO2 nanosheets absorbed with 1.0 mg per ml vs. with 10 mg per ml bovine serum protein and then vacuum filtered). Degradation profile of different scaffolds obtained by measuring time-dependent manganese concentrations in the solution using Inductively coupled plasma mass spectrometry (ICP-MS, Fisons Instruments PlasmaQuad 2+). Each sample was measured 3 times to obtain error bars and standard deviation. To control scaffold degradation without any additional bioreductants, different cell densities (0, 0.1, 0.5, 1, and 5 million cells per well in a 24 well plate) were seeded onto MnO2 nanoscaffold, and the complete degradation time was monitored by the full disappearance of dark color. 0.5 ml media was changed every two days. Each experiment was repeated twice. Results are summarized in Supplementary Fig. 10m.
Fabrication and characterization of MnO2 hybrid nanoscaffold
To fabricate the MnO2 hybrid nanoscaffolds, 10 ml of MnO2 nanosheet solution at a specific concentration was filtered through a cellulose filter paper (pore size = 20 nm) under the vacuum condition. Then the filter paper was taken out and cut into sizes and shapes of choice. To transfer into a transparent glass substrate, cleaned glass was first treated with oxygen plasma, then the MnO2 nanosheet deposited on filter paper was wetted with de-ionized water and pressed against the glass. A 2.0 kg per cm2 pressure was placed on top of the filter paper for 8–12 h, and glass attached with MnO2 nanosheet was detached from the weight. To remove the cellulose attached with nanoscaffold, the substrate was incubated in acetone for 0.5 h and then briefly washed in methanol for 1 h. The transparency of nanoscaffold can be easily tuned by using different concentrations of MnO2 nanosheet solution. The 3 concentrations used in Supplementary Fig. 3 are 50 μg per ml, 100 μg per ml and 200 μg per ml. For cellular studies, the concentration of 200 μg per ml MnO2 nanosheet solution was used throughout the study. Graphene oxide assembled scaffold was fabricated using an identical protocol with a graphene oxide aqueous solution of 200 μg per ml. FESEM (Field Emission Scanning Electron Microscopy, Zeiss with Oxford EDS) was used to characterize the nanoscaffold.
To form MnO2 laminin hybrid nanoscaffold, 400 μL of MnO2 nanosheet aqueous solution (2 mg per ml) was quickly added to 100 μL of laminin solution (1.0 mg per ml, PBS, PH = 7.4). Then the laminin conjugated MnO2 nanosheet was centrifuged and re-suspended in 10 ml de-ionized water (MnO2 nanosheet concentration was 80 μg per ml). After vacuum filtration, the cellulose filter paper or PCL fiber was cut into size and shape of interest for the following cell studies.
HiPSC-NSC culture
Human iPSC-NPCs were derived from human iPSCs (WT126 clone 8; and WT33 clone 1)57. iPSC-NPCs were expanded in a proliferation media containing DMEM/F12 with Glutamax (Invitrogen), B27-supplement (Invitrogen), N2 (Stem Cells), and 20 ng per mL FGF2 (Invitrogen). Tissue culture vessels were treated with Matrigel (Corning) 1:200 dilution with DMEM (Invitrogen) at 37 °C for 1 h. To initiate the neuronal differentiation process, bFGF was removed. Fresh media was exchanged every other day. iPSC-NSCs with passage 8–11 were used in all our transplantation and in vitro studies.
Differentiation of iPSC-NSC on MnO2 nanoscaffold
The viabilities of iPSC-NSCs and human neural progenitor cells (hNPC, Supplementary Methods) cultured on MnO2 nanoscaffold were measured by presto blue cell viability assay (Thermo Fisher, Catalog No.: A13261, 10% volume ratio as compared to cell media). Into 24-well plates, laminin (10 μg/ml) was first coated onto the glass (control), graphene oxide assembled scaffold (positive control) and MnO2 nanoscaffold at a concentration of 20 μg per ml (media volume: 1.0 ml per well), for 4 h. Then the iPSC-NSC was seeded into each well at a cell density of 20 k in growth media (bFGF added, 20 ng per ml). After the cells were cultured for 48 h, cell viabilities cultured on different substrates were quantified using fluorescence (excitation at 570 nm and emission at 590 nm) presto blue assay and normalized to the control group (glass). For the neurite length analysis, neurites on each substrate were first automatically traced and the lengths were automatically measured by NeuronJ in ImageJ software. The values are all averaged from 9–12 measurements in the representative immunostaining images58. Here is a summary of the average neurite lengths with standard deviation obtained from software: Glass control, 5.2 ± 2.3 μm; MnO2 nanoscaffold, 59.3 ± 19.8 μm; MnO2 laminin nanoscaffold, 84.5 ± 26.5 μm.
For the differentiation of iPSC-NSC on glass substrates, MnO2 nanoscaffold, graphene oxide assembled scaffold (GO nanoscaffold) and glass were first sterilized in the ultraviolet (UV) lamp for 5 min and then coated with laminin solution (10 μg per ml) for 4 h. The substrates were placed in 24-well plates, and iPSC-NSCs were seeded into the wells at a cell density of 60 k cells per well. The cells proliferated for 24 h, and the media was changed to differentiation media without bFGF. To observe the stem cell proliferation and attachment onto the substrate, the cells were imaged in the optical microscope (Nikon Eclipse Ti-E microscope). After 6-days’ differentiation, the cells were fixed and immunostained with nuclei (Hoechst, Thermo Fisher, catalog number: 33346, 1:100 dilution, 0.2 mM) and neuronal marker (TuJ1, Cell Signaling, catalog number: 4466, 1:500 dilution). To quantify the neuronal markers (TuJ1) and astrocyte markers (GFAP), qRT-PCR was conducted by using GAPDH mRNA as a control (Supplementary Table 2).
Fabrication of MnO2 laminin hybrid nanoscaffold
MnO2 laminin hybrid nanoscaffold can be facilely fabricated by adding 10 μL of MnO2 nanosheet aqueous solution (3 mg per ml) into 100 μL laminin solution (1 mg per ml), and the MnO2 nanosheet will be assembled within 5 s. To fabricate larger scale MnO2 laminin hybrid nanoscaffold, 100 μL of MnO2 nanosheet aqueous solution (3 mg per ml) was added into 500 μL laminin solution (1 mg per ml) and then vacuum filtered on a cellulose paper as described above. The structure of MnO2 laminin hybrid nanoscaffold was then analyzed in FESEM. To fabricate cell encapsulated MnO2 laminin-nanoscaffold, 1 million iPSC-NSCs were centrifuged down and re-dispersed in 25 μL laminin PBS solution. Different amount (0, 0.3, 1.5, 3, 15, and 30 μL) of MnO2 nanosheet solution (3 mg per ml) was injected into the cell laminin solution, and a iPSC-NSC encapsulated pellet was spontaneously formed after one hour. To investigate the interaction between MnO2 and encapsulated iPSC-NSCs, the medium was removed and the neurons were fixed in Formalin solution (Sigma-Aldrich) followed by two PBS washes. The biological samples (prepared under 15 μL MnO2 condition) were then dehydrated to eliminate water through a series of ethanol dehydration process by replacing PBS with 50% ethanol/water, 70% ethanol/water, 85% ethanol/water, 95% ethanol/water, and absolute ethanol twice for 10 min each in succession. The biological samples were then stored in absolute ethanol before transferring to critical point dryer to eliminate traces of ethanol. Then 20 nm of gold was sputter coated onto the surface of biological samples after drying. FESEM was then used for micrograph acquisition.
For the differentiation of iPSC-NSC on substrates, glass, MnO2 nanoscaffold and MnO2 laminin hybrid nanoscaffold were first sterilized in the UV lamp for 5 min and then coated with laminin solution (10 μg per ml) for 4 h. The substrates were placed in 24-well plates, and iPSC-NSCs were seeded into the wells at a cell density of 60,000 per well. After 6 days’ differentiation, the cells were fixed, and immunostaining on nuclei (DAPI) and neuronal marker (TuJ1) was conducted. Stem cell assays were repeated 3 times to obtain statistical information unless mentioned otherwise. Student t-test was used for two group analysis and ANOVA with Tukey post hoc test was used for multi-group (more than 3 groups) analysis. For the long-term (1 month, 30 days) stem cell differentiation assay, identical protocol was used with twice media change per week. Mature neuronal marker (MAP2) was used for identifying neurons differentiated from iSPC-NSCs on MnO2 nanoscaffold.
Calcium imaging of neurons differentiated from iPSC-NSCs on MnO2 laminin hybrid nanoscaffold in 12 well-plates. iPSC-NSCs were differentiated on MnO2 laminin hybrid nanoscaffold using an identical protocol mentioned above for 6 days, then the cells were incubated with 1 ml of Fura-2 AM (Life Technologies, Catalog Number: F1201, 1:200 dilution, 5 μg per ml) in cell media for 1 h. Afterwards, cell media was changed to PBS. Under the movie mode of a fluorescence microscope, concentrated KCl solution in PBS (50 mM, 0.1 ml) was added to the cells, and the movie was taken for 10 min with 60 frames per seconds. The movies were pseudocolored, with red indicating strong calcium flux and green indicating weak calcium flux. An identical procedure was also applied for collecting calcium imaging of neurons differentiated from hNPCs. A summary of time dependent calcium intensity peaks can be found in Fig. 4, which was automatically obtained from the Nikon ND2 software.
Dye loading on MnO2 nanoscaffold and MRI studies
To study drug loading and release on MnO2 nanoscaffold, rhodamine B was used a model drug. Briefly, 0.3 mg rhodamine B (Alfa Aesar, Catalog Number: A13572) was added into 3.0 ml of MnO2 nanosheet solution. After incubation at room temperature for 12 h, 5.0 ml PBS (PH = 7.4) was gradually added into the solution and RhB loaded MnO2 nanosheet was centrifuged down at 3431 g for 5 min and extensively washed with PBS for 6 times to remove the residual RhB solution. Then the RhB-loaded MnO2 nanosheet was re-suspended in 10 ml solution and re-assembled with laminin using the identical conditions for fabricating MnO2 laminin hybrid nanoscaffolds. To monitor the dye hold-up, RhB-nanoscaffold was incubated with PBS for 12 h, then the fluorescence of the supernatant was detected by a fluorescence spectra (Varian Cary Eclipse). The dye loading was confirmed by degrading the RhB-nanoscaffold using 1.0 mg/ml ascorbic acid PBS solution. Instant appearance of pink color from RhB proves the loading of RhB inside nanoscaffold. RhB-nanoscaffold before and after degradation was also spotted in a glass slide in a close-proximity and then imaged in the fluorescent microscope. To test the correlation between MRI signals and RhB released, different amount of RhB-nanoscaffold [5, 2.5, 1, 0.5, 0.1 mg (from left to right in Fig. 5c)] were degraded with ascorbic acid (1.0 mg per ml) to form a homogeneous solution. Then the same solution in 96-well plates was used for MRI (Aspect’s M2TM Compact High-Performance MRI, 1T) measurement and fluorescence measurement under Nikon fluorescent microscope.
To study the day-dependent drug (RhB) release from our MnO2-laminin hybrid nanoscaffold, PBS with 10 μg per ml vitamin C was used to incubate the RhB loaded nanoscaffold, and was changed regularly every day. Fluorescence images (Nikon fluorescent microscope) were taken at Day1, Day2, and Day7, and the intensities from 3 different experiments were used to quantify the amount of RhB released. As a control, PCL polymer was dissolved with RhB and then formed a scaffold by drying at room temperature. Then the dye release was measured at the same time points as RhB loaded nanoscaffolds. The percentage of dye release was all normalized to the fluorescence intensity obtained at Day1.
To load neurogenic drugs into MnO2 laminin hybrid nanoscaffold, DAPT (N-[(3,5-Difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester, Tocris, Catalog Number: 2634) was first dissolved in a PBS: DMF = 9:1 solution (dimethylformamide, Sigma-Aldrich) at a concentration of 0.1 mg per ml. Then 1.0 ml of DAPT solution was quickly mixed with 100 μL of 3 mg per ml MnO2 nanosheet aqueous solution. After incubating for 12 h, the solution was centrifuged down and washed with de-ionized water for 3 times. The successful loading of DAPT onto MnO2 nanosheet was confirmed by MALDI-TOF (Bruker, Ultraflex) based on the Na+-DAPT peak at 455 (molecular weight to charge ratio). Briefly, 50 μL MnO2 nanosheet loaded with DAPT solution was mixed with 50 μL gold nanoparticle solution (Ted Pella, 10 nm, 5.7 × 1012 particles per ml). Then 1 μL of the mixed solution was drop-cast onto ITO glass and baked at 50 °C for 1 min to fully evaporate water. The DAPT solution was drop-cast on the same ITO glass as a reference. ITO glass was placed into the MALDI-TOF and exposed with a laser for the analysis.
To measure the DAPT loading and releasing profile on DAPT MnO2 nanoscaffold, 3.0 mg MnO2 nanosheets were first loaded with DAPT using the previous protocol and then assembled into MnO2 laminin hybrid nanoscaffolds. Using UV-Vis spectroscopy, we first identified the characteristic absorption peak of DAPT (20 μM in water with 1% DMF) at 264 nm. Then the drug loading amount was determined by the full disappearance of the 264 nm peak after incubated with nanosheets (2.0 ml PBS, 1.5 mg per ml). This corresponds to a loading efficiency of 110 μg per 3.0 mg nanosheets and a molar ratio between DAPT and manganese atom = 1:134 (Supplementary Fig. 13). After that, to quantify the release of DAPT, degradation and DAPT release from the nanoscaffold was initiated by incubating it in a solution containing 10 μg per ml ascorbic acid. As DAPT form strong binding complex with MnO2 nanosheets (Binding Energy = −18.43 kcal per mol), we monitored DAPT release through quantifying the amount of manganese amount at different time points, and estimated the drug release based on the constant molar ratio between DAPT and manganese (1:134) in the MnO2-DAPT complex. The average daily release was quantified through dividing the total amount of manganese released by the length of degradation. The summarized DAPT release profile can be found in Supplementary Fig. 13.
DFT calculations on small molecule and MnO2 binding
DFT calculations were carried out using the Quantum ESPRESSO software package. For the geometry optimization Perdew-Burke-Ernzerhof (PBE) functional along with D2 dispersion corrections were used59,60. The MnO2 surface and the MnO2 bound complexes were treated with DFT + U method. This is because conventional DFT functionals are unable to describe the strong correlation effect among the partially filled d states in Mn61. The Hubbard parameter ‘U’, is introduced for the Mn 3d electrons to describe the on-site Coulomb interaction, as given in the well-known GGA + U method62. The values of U = 4 eV and J = 0 eV for MnO2 were adopted63. Spin-polarized calculations were performed since bulk MnO2 has an antiferromagnetic ground state. The electron cores were defined using ultrasoft pseudopotential for all the elements and were extracted from the Quantum ESPRESSO main website (http://theossrv1.epfl.ch/Main/Pseudopotentials). For the k-point mesh, a γ-center was used. The wave function cutoff of 60 Ry and kinetic energy cutoff of 240 Ry were used in all the cases studied. The Gaussian smearing was turned so that the difference between the free energy and the total energy is less than 0.005 Ry per atom. The energy convergence was set to 1 × 10−6 a.u. and the force convergence threshold for the ionic minimization was set at 1 × 10−4 a.u.
The binding energies on the MnO2 surface were calculated for a series of small molecules (Supplementary Table 1) and the DAPT drug molecule. The size of the cell was taken equivalent to the size of the MnO2 surface that has 8 × 8 oxygen atoms at the periphery. The box size for the simulated system is 23 × 23 × 40 Å, and periodic boundary conditions are used. This condition was chosen to mimic the 2D MnO2 surface. We first performed geometry optimizations for the bound complexes, with the resulting energy referred to as Ecomplex. We then optimized the structures of isolated MnO2 surface and the molecule of interest, obtaining their energies EMnO2 and Emol, respectively. The binding energy is defined as \(E_{\mathrm b} = E_ {\rm complex} - E_{\rm MnO_2} - E_{\rm mol}\). Negative Eb indicates binding while positive Eb indicates repulsion to the surface.
In vivo transplantation of hiPSC-NSCs
Spinal cord injury, transplantation of nanoscaffold and tissue harvest: All animal work was conducted following the regulation of the Institutional Animal Care and Use Committee (IACUC) at Rutgers University. The Notch1CR2-GFP transgenic mouse (Mus musculus) was used in this study. Adult mice that are 5–6 months old were picked for the spinal cord injury experiments. No difference was observed between male or female animals, and thus the gender was not specified. Animals are randomized without pre-knowledge of their behaviors and then assigned to different experimental groups without selection. Three mice were used for each group. Observers were blind to animal groups when performing experiments and analysis. During the surgery, initial anesthetization was performed with 5% isoflurane and then maintained at 2% Isoflurane. For hemisection, a laminectomy at T10~11 lateral section of spinal cord (length of gap ≈1 mm) was first performed. Then the dorsal blood vessel was burned with a cauterizer, and the spinal cord was cut from middle line toward the left using a #10 scalpel. Following induction of injury, bio-materials with iPSC-NSCs, laminin-coated and DAPT-treated PCL, or surgifoam was inserted into the wound site, and the muscle around surgical wound was sutured and skin is stapled using wound clips. A cell density of 1 million cells/cm2 was used for transplantation and mice were sacrificed 7-week after injury. For harvest, the spinal cords from the injured animals were obtained via microsurgical dissection. They were washed in 1× PBS and fixed with 4% (w/v) paraformaldehyde (PFA) for 24 h. Fixed tissues were washed again and then cryopreserved in 30% (w/v) sucrose for 48 h. Afterwards, the spinal cord tissue was embedded in cryo-preserving media (Tissue Tek® OCT compound) and kept frozen at −80 °C. Spinal cord sections were stained with PH3, GFAP, and F4/80 antibodies to analyze the long-term effect of nanoscaffold on SCI (Fig. 8). One-way ANOVA was used for multi-group analysis. Data represents mean ± s.d., n = 3, *P < 0.05, **P < 0.01 with Tukey’s post-hoc analysis. All tissue sections are in the center of the scaffold implants and then identified nearby the transplanted sites.
In vivo hiPSC-NSC-GFP transplantation assay
To study enhanced transplantation of iPSC-NSCs using our nanoscaffold, we further transplanted GFP labeled iPSC-NSC cells (iPSC-NSC-GFP) into a non GFP wild type C57BL/6 mouse strain64. To obtain GFP labeled cells, we transfected iPSC-NSCs with plasmid expressing EGFP using previously reported protocol (Supplementary Fig. 20). We confirmed the high transfection efficiency (>90%) and strong green fluorescence from iPSC-NSCs before seeded to the scaffold for in vivo cell transplantation using fluorescent microscope (Supplementary Fig. 20b). The surgical procedures for transplantation of iPSC-NSC-GFP into C57BL/6 were kept identical as the experiments on Notch1CR2-GFP mice and we repeated the immuno staining on tissue sections 1-week post transplantation and 1-month (30 days) post transplantation. In addition to the 3 groups [injury only (surgifoam insertion), 3D BHI nanoscaffold, and PCL cell group] evaluated in Notch1CR2 mice, we added 3 other important control groups to better support the therapeutic potential of our developed scaffold system: MnO2 scaffold without laminin or DAPT but with cell transplantation (MnO2 cell group), MnO2 nanoscaffold with laminin and DAPT but without cell transplantation (MnO2 DAPT group), and direct injection of GFP iPSC-NSCs with laminin (laminin cell group). All other conditions were kept identical to the experimental group (3D BHI nanoscaffold). Each group includes 3 animals (n = 3) to check reproducibility. We summarized the animal groups in Supplementary Fig. 20, and all the tissue analysis results were summarized in Fig. 7 and Supplementary Figs. 21–23. GFP, TuJ1 and MAP2 positive cells were first identified by automatic detection function in the NIS Nikon software (NIS element AR) then the amount of cells were recorded for making the graphs (Supplementary Methods). Percentage of Syn positive cells are quantified by first identify GFP + Syn + cells then divided by amount of GFP + cells in each section. Unpaired student t-test was used for two group significance analysis and one-way ANOVA was used for multi-group analysis. Data represents mean ± s.d., n = 3 unless described otherwise, *P < 0.05, **P < 0.01, ***P < 0.001. In Supplementary Fig. 20, when we count GFP + cells, we used the automatic detection function in the NIS Nikon software to identify GFP + cells shown in Supplementary Fig. 20e, then summarized the amount of GFP + cells at specific distance intervals (100 μm) in the sagittal sections. All tissue sections are in the center of the scaffold implants and then identified nearby the transplanted sites.
Data availability
The data that support the findings of this study are available from the authors on reasonable request. New data related to this manuscript can also be found on the following link: https://figshare.com/projects/A_Biodegradable_Hybrid_Inorganic_Nanoscaffold_for_Advanced_Stem_Cell_Therapy/29040.
Change history
05 September 2018
This Article was originally published without the accompanying Peer Review File. This file is now available in the HTML version of the Article; the PDF was correct from the time of publication.
References
Rubiano, A. M., Carney, N., Chesnut, R. & Puyana, J. C. Global neurotrauma research challenges and opportunities. Nature 527, S193–S197 (2015).
Tran, A. P. & Silver, J. Neuroscience. Systemically treating spinal cord injury. Science 348, 285–286 (2015).
Bouton, C. E. et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 533, 247 (2016).
Lu, P. et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150, 1264–1273 (2012).
Ruschel, J. et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348, 347–352 (2015).
van den Brand, R. et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 (2012).
Tsuji, O. et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl Acad. Sci. USA 107, 12704–12709 (2010).
McDonald, J. W. et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 5, 1410–1412 (1999).
Lindvall, O. & Kokaia, Z. Stem cells for the treatment of neurological disorders. Nature 441, 1094–1096 (2006).
Park, K. I., Teng, Y. D. & Snyder, E. Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 20, 1111–1117 (2002).
Thuret, S., Moon, L. D. & Gage, F. H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7, 628 (2006).
Kan, E. M., Ling, E. A. & Lu, J. Stem cell therapy for spinal cord injury. Curr. Med. Chem. 17, 4492–4510 (2010).
Griffin, D. R., Weaver, W. M., Scumpia, P. O., Di Carlo, D. & Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 14, 737–744 (2015).
Huebsch, N. et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 14, 1269–1277 (2015).
Murphy, W. L., McDevitt, T. C. & Engler, A. J. Materials as stem cell regulators. Nat. Mater. 13, 547–557 (2014).
Carlson, A. L. et al. Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds. Nat. Commun. 7, 10862 (2016).
Zhang, B. et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 15, 669–678 (2016).
Green, J. J. & Elisseeff, J. H. Mimicking biological functionality with polymers for biomedical applications. Nature 540, 386–394 (2016).
Kang, H.-W. et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34, 312–319 (2016).
DeForest, C. A. & Tirrell, D. A. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater. 14, 523–531 (2015).
DeForest, C. A., Polizzotti, B. D. & Anseth, K. S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 8, 659–664 (2009).
Luo, Y. & Shoichet, M. S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 3, 249–253 (2004).
Shah, S. et al. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv. Mater. 26, 3673–3680 (2014).
Kim, T. H. et al. Controlling differentiation of adipose-derived stem cells using combinatorial graphene hybrid-pattern arrays. ACS Nano 9, 3780–3790 (2015).
Wang, Y. et al. Fluorinated graphene for promoting neuro-induction of stem cells. Adv. Mater. 24, 4285–4290 (2012).
Park, S. Y. et al. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 23, 263–267 (2011).
Laurenti, M. et al. Two-dimensional magnesium phosphate nanosheets form highly thixotropic gels that up-regulate bone formation. Nano. Lett. 16, 4779–4787 (2016).
Aldinucci, A. et al. Carbon nanotube scaffolds instruct human dendritic cells: modulating immune responses by contacts at the nanoscale. Nano. Lett. 13, 6098–6105 (2013).
Dvir, T. et al. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 6, 720–725 (2011).
Dvir, T., Timko, B. P., Kohane, D. S. & Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nano 6, 13–22 (2011).
Chueng, S.-T. D., Yang, L., Zhang, Y. & Lee, K.-B. Multidimensional nanomaterials for the control of stem cell fate. Nano Converg. 3, 23 (2016).
Zhao, Z. L. et al. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet-aptamer nanoprobe. J. Am. Chem. Soc. 136, 11220–11223 (2014).
Chen, Y. et al. Break-up of two-dimensional MnO2 nanosheets promotes ultrasensitive pH-triggered theranostics of cancer. Adv. Mater. 26, 7019 (2014).
Deng, R. R., **e, X. J., Vendrell, M., Chang, Y. T. & Liu, X. G. Intracellular glutathione detection using MnO2-nanosheet-modified upconversion nanoparticles. J. Am. Chem. Soc. 133, 20168–20171 (2011).
Dalby, M. J., Gadegaard, N. & Oreffo, R. O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 13, 558–569 (2014).
Wang, S. et al. A nanostructured molybdenum disulfide film for promoting neural stem cell neuronal differentiation: toward a nerve tissue-engineered 3D scaffold. Adv. Biosyst. 1, 1600042 (2017). https://onlinelibrary.wiley.com/doi/abs/10.1002/adbi.201600042
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
López‐Dolado, E. et al. Subacute tissue response to 3D graphene oxide scaffolds implanted in the injured rat spinal cord. Adv. Healthc. Mater. 4, 1861–1868 (2015).
Halliwell, B. & Gutteridge, J. M. C. The antioxidants of human extracellular fluids. Arch. Biochem. Biophys. 280, 1–8 (1990).
Khetan, S. et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12, 458–465 (2013).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Yu, X., Dillon, G. P. & Bellamkonda, R. V. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tiss. Eng. 5, 291–304 (1999).
Wang, Q. et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 463, 339–343 (2010).
O’Neill, H. S. et al. Biomaterial-enhanced cell and drug delivery: lessons learned in the cardiac field and future perspectives. Adv. Mater. 28, 5648–5661 (2016).
Tam, R. Y., Fuehrmann, T., Mitrousis, N. & Shoichet, M. S. Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology 39, 169–188 (2014).
Hollister, S. J. Porous scaffold design for tissue engineering. Nat. Mater. 4, 518–524 (2005).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 16071 (2016).
Choi, J.-s. et al. Distance-dependent magnetic resonance tuning as a versatile MRI sensing platform for biological targets. Nat. Mater. 16, 537–542 (2017).
El Bejjani, R. & Hammarlund, M. Notch signaling inhibits axon regeneration. Neuron 73, 268–278 (2012).
Androutsellis-Theotokis, A. et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 442, 823–826 (2006).
Tzatzalos, E. et al. A cis-element in the Notch1 locus is involved in the regulation of gene expression in interneuron progenitors. Dev. Biol. 372, 217–228 (2012).
Lu, P. et al. Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. J. Clin. Invest. 127, 3287–3299 (2017).
Führmann, T. et al. Combined delivery of chondroitinase ABC and human induced pluripotent stem cell-derived neuroepithelial cells promote tissue repair in an animal model of spinal cord injury. Biomed. Mater. 13, 024103 (2018).
Kai, K. et al. Room-temperature synthesis of manganese oxide monosheets. J. Am. Chem. Soc. 130, 15938–15943 (2008).
Kim, T.-H. et al. Controlling differentiation of adipose-derived stem cells using combinatorial graphene hybrid-pattern arrays. ACS Nano 9, 3780–3790 (2015).
Shah, S. et al. Guiding stem cell differentiation into oligodendrocytes using graphene‐nanofiber hybrid scaffolds. Adv. Mater. 26, 3673–3680 (2014).
Marchetto, M. C. N. et al. A model for neural development and treatment of rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010).
Meijering, E. et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytom. Part A 58, 167–176 (2004).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396–1396 (1997).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 19 (2010).
Wang, L., Maxisch, T. & Ceder, G. Oxidation energies of transition metal oxides within the GGA + U framework. Phys. Rev. B 73, 6 (2006).
Wang, Y. C., Chen, Z. H. & Jiang, H. The local projection in the density functional theory plus U approach: a critical assessment. J. Chem. Phys. 144, 9 (2016).
Sun, C. H., Wang, Y., Zou, J. & Smith, S. C. A formation mechanism of oxygen vacancies in a MnO2 monolayer: a DFT + U study. Phys. Chem. Chem. Phys. 13, 11325–11328 (2011).
Luchetti, S. et al. Comparison of immunopathology and locomotor recovery in C57BL/6, BUB/BnJ, and NOD-SCID mice after contusion spinal cord injury. J. Neurotrauma 27, 411–421 (2010).
Acknowledgements
We acknowledge financial support from the NIH Director’s Innovator Award (1DP20D006462-01), NIH R01 (1R01DC016612-01), NIH R21 (1R21NS085569-01), New Jersey Commission on Spinal Cord (CSCR17IRG010, CSR13ERG005 (K.B.L), and 15IRG006 (L.C.)), NSF CHE-1429062, CBET-1236508, American Chemical Society New Directions Award (PRF# 55869-ND10), and the University City Science Center’s QED Award. M.P. and Y.L. acknowledge financial support from Graduate Training in Emerging Areas of Precision and Personalized Medicine (P200A150131) and NJCSCR [12FEL001], respectively. G.D. and L.W. acknowledge the Office of Advanced Research Computing at Rutgers for providing access to the Amarel cluster. The calculations have also used the Extreme Science and Engineering Discovery Environment supported by NSF (ACI-1548562). We would like to acknowledge Thanapat Pongkulapa, Prof. Gene Hall and Rutgers Molecular Imaging Center (RUMIC) for graciously hel** us with PCR analysis, providing us access to the MALDI-TOF instruments and MRI imaging facilities respectively. We would also like to acknowledge Ning Chiang, Prof. Dongming Sun, and Prof. Wise Young for kindly providing us GFP labeled iPSC-NSCs for our in vivo experiments. Additionally, we are also grateful to Dr. Shreyas Shah, Brian Conley, and Kaixiong Tu for their valuable advices and scientific discussions during manuscript preparation.
Author information
Authors and Affiliations
Contributions
L.Y. and K.B.L. conceived and designed the experiments. S.T.D.C. and C.R. contributed to the cell studies and electron microscopy analysis. L.Y. performed the experiments. Y.L., M.P., and L.C. contributed to in vivo experiments and image analysis. G.D. and L.W. contributed to DFT calculations on drug-scaffold interaction. L.Y. and K.B.L analyzed the data. L.Y. and K.B.L wrote the manuscript. The principle investigator is K.B.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, L., Chueng, ST.D., Li, Y. et al. A biodegradable hybrid inorganic nanoscaffold for advanced stem cell therapy. Nat Commun 9, 3147 (2018). https://doi.org/10.1038/s41467-018-05599-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-018-05599-2
- Springer Nature Limited
This article is cited by
-
Phototoxicity-free blue light for enhancing therapeutic angiogenic efficacy of stem cells
Cell Biology and Toxicology (2023)
-
Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis
Nature Communications (2022)
-
Exploring the configuration spaces of surface materials using time-dependent diffraction patterns and unsupervised learning
Scientific Reports (2020)
-
A dual-modal colorimetric and photothermal assay for glutathione based on MnO2 nanosheets synthesized with eco-friendly materials
Analytical and Bioanalytical Chemistry (2020)
-
Generation of uniform-sized multicellular tumor spheroids using hydrogel microwells for advanced drug screening
Scientific Reports (2018)