Abstract
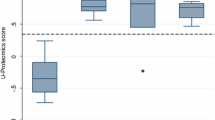
Cardiovascular disease (CVD) affects individuals across the lifespan, with multiple cardiovascular (CV) risk factors increasingly present in young populations. The underlying mechanisms in early cardiovascular disease development are complex and still poorly understood. We therefore employed urinary proteomics as a novel approach to gain better insight into early CVD-related molecular pathways based on a CVD risk stratification approach. This study included 964 apparently healthy (no self-reported chronic illnesses, free from clinical symptoms of CVD) black and white men and women (aged 20–30 years old) from the African Prospective study on the Early Detection and Identification of Cardiovascular disease and Hypertension (African-PREDICT) study. Cardiovascular risk factors used for stratification included obesity, physical inactivity, tobacco use, high alcohol intake, hyperglycemia, dyslipidemia and hypertension. Participants were divided into low (0 risk factors), medium (1–2 risk factors) and high (≥3 risk factors) CV risk groups. We analyzed urinary peptidomics by capillary electrophoresis time-of-flight mass spectrometry. After adjusting for ethnicity, sex and age, 65 sequenced urinary peptides were differentially expressed between the CV risk groups (all q-values ≤ 0.01). These peptides included a lower abundance of collagen type I- and III-derived peptides in the high compared to the low CV risk group. With regard to noncollagen peptides, we found a lower abundance of alpha-1-antitrypsin fragments in the high compared to the low CV risk group (all q-values ≤ 0.01). Our findings indicate lower abundances of collagen types I and III in the high compared to the low CV risk group, suggesting potential early alterations in the CV extracellular matrix.


Similar content being viewed by others
References
Gooding HC, Gidding SS, Moran AE, Redmond N, Allen NB, Bacha F, et al. Challenges and Opportunities for the Prevention and Treatment of Cardiovascular Disease Among Young Adults: Report From a National Heart, Lung, and Blood Institute Working Group. J Am Heart Assoc. 2020;9:e016115.
Clark CJ, Alonso A, Spencer RA, Pencina M, Williams K, Everson-Rose SA. Predicted long-term cardiovascular risk among young adults in the national longitudinal study of adolescent health. Am J Public Health. 2014;104:e108–e115.
Kruger R, Litwin M, Climie RE. Editorial: Determinants and Impact of Early Vascular Aging in Children and Adolescents. Front Pediatr. 2022; 10. Editorial. https://doi.org/10.3389/fped.2022.871524.
Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circ J. 2017;136:e393–e423.
Gao Z, Chen Z, Sun A, Deng X. Gender differences in cardiovascular disease. Med Nov Technol Devices. 2019;4:100025.
Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18:109–14.
Doran S, Arif M, Lam S, Bayraktar A, Turkez H, Uhlen M, et al. Multi-omics approaches for revealing the complexity of cardiovascular disease. Briefings Bioinf. 2021; 22. https://doi.org/10.1093/bib/bbab061.
MacLellan WR, Wang Y, Lusis AJ. Systems-based approaches to cardiovascular disease. Nat Rev Cardiol. 2012;9:172–84.
Aslam B, Basit M, Nisar MA, Khurshid M, Rasool MH. Proteomics: technologies and their applications. J Chromatogr Sci. 2017;55:182–96.
Pejchinovski M, Hrnjez D, Ramirez‐Torres A, Bitsika V, Mermelekas G, Vlahou A, et al. Capillary zone electrophoresis on‐line coupled to mass spectrometry: a perspective application for clinical proteomics. PROTEOMICS–Clin Appl. 2015;9:453–68.
Gramolini AO, Emili A. Uncovering early markers of cardiac disease by proteomics: avoiding (heart) failure! Expert Rev Proteom. 2005;2:631–4.
Delles C, Schiffer E, von Zur Muhlen C, Peter K, Rossing P, Parving HH, et al. Urinary proteomic diagnosis of coronary artery disease: identification and clinical validation in 623 individuals. J Hypertens. 2010;28:2316–22.
Zimmerli LU, Schiffer E, Zürbig P, Good DM, Kellmann M, Mouls L, et al. Urinary proteomic biomarkers in coronary artery disease. Mol Cell Proteom. 2008;7:290–8.
Verbeke F, Siwy J, Van Biesen W, Mischak H, Pletinck A, Schepers E, et al. The urinary proteomics classifier chronic kidney disease 273 predicts cardiovascular outcome in patients with chronic kidney disease. Nephrol Dial Transpl. 2019. https://doi.org/10.1093/ndt/gfz242.
Øvrehus MA, Zürbig P, Vikse BE, Hallan SI. Urinary proteomics in chronic kidney disease: diagnosis and risk of progression beyond albuminuria. Clin Proteom. 2015;12:21–21.
Mischak H, Kaiser T, Walden M, Hillmann M, Wittke S, Herrmann A, et al. Proteomic analysis for the assessment of diabetic renal damage in humans. Clin Sci. 2004;107:485–95.
Rossing K, Mischak H, Dakna M, Zürbig P, Novak J, Julian BA, et al. Urinary proteomics in diabetes and CKD. J Am Soc Nephrol. 2008;19:1283–90.
Zhang ZY, Ravassa S, Nkuipou‐Kenfack E, Yang WY, Kerr SM, Koeck T, et al. Novel Urinary Peptidomic Classifier Predicts Incident Heart Failure. J Am Heart Assoc. 2017;6:e005432.
Schutte AE, Gona PN, Delles C, Uys AS, Burger A, Mels CM, et al. The African Prospective study on the Early Detection and Identification of Cardiovascular disease and Hypertension (African-PREDICT): Design, recruitment and initial examination. Eur J Prev Cardiol. 2019;26:458–70.
Stewart A, Marfell-Jones M, Olds T, Ridder DH. International standards for anthropometric assessment. ISAK 2011: 50-53. ISBN: 0-620-36207-3.
Patro BK, Jeyashree K, Gupta PK. Kuppuswamy’s socioeconomic status scale 2010—the need for periodic revision. Indian J Pediatr. 2012;79:395–6.
Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. JPAH. 2009;6:790–804.
Barna I, Keszei A, Dunai A. Evaluation of Meditech ABPM-04 ambulatory blood pressure measuring device according to the British Hypertension Society protocol. Blood Press Monit. 1998;3:363–8.
Mavrogeorgis E, Mischak H, Latosinska A, Siwy J, Jankowski V, Jankowski J. Reproducibility evaluation of urinary peptide detection using CE-MS. Molecules. 2021;26:7260.
Mischak H, Vlahou A, Ioannidis JP. Technical aspects and inter-laboratory variability in native peptide profiling: The CE–MS experience. Clin Biochem. 2013;46:432–43.
Albalat ABVZP, Siwy J, Mullen W. High-resolution proteome/peptidome analysis of body fluids by capillary electrophoresis coupled with MS. Methods Mol Biol. 2013;984:153–65.
Good DM, Zürbig P, Argilés À, Bauer HW, Behrens G, Coon JJ, et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteom. 2010;9:2424–37.
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Faul FEE, Lang AG, Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Yoo EG. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J Pediatr. 2016;59:425.
Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2021. PMID: 31082114.
Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13:1236.
Du Buisson K, Kramer S. Ampath medical surveillance guideline. 2014-2015. URL: https://www.medichem.org/information/Ampath%202014.pdf.
Puukka K, Hietala J, Koivisto H, Anttila P, Bloigu R, Niemelä O. Age-related changes on serum ggt activity and the assessment of ethanol intake. Alcohol Alcohol. 2006 Sep;41:522–7.
American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes care. 2018;41:S13–S27.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Russ J Cardiol. 2020;25:3826.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39:3021–104.
Nehring SM, Goyal A, Patel BC. C Reactive Protein. In: StatPearls. Treasure Island (FL): StatPearls. 2021.
Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58.
Kuznetsova T, Mischak H, Mullen W, Staessen JA. Urinary proteome analysis in hypertensive patients with left ventricular diastolic dysfunction. Eur Heart J. 2012;33:2342–50.
Magalhaes P, Pontillo C, Pejchinovski M, Siwy J, Krochmal M, Makridakis M, et al. Comparison of urine and plasma peptidome indicates selectivity in renal peptide handling. Proteom Clin Appl. 2018;12:1700163.
He T, Pejchinovski M, Mullen W, Beige J, Mischak H, Jankowski V. Peptides in plasma, urine, and dialysate: toward unravelling renal peptide handling. Proteom Clin Appl. 2021;15:2000029.
McNulty M, Mahmud A, Spiers P, Feely J. Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects. J Hum Hypertens. 2006;20:867–73.
Alkhalaf A, Zürbig P, Bakker SJ, Bilo HJ, Cerna M, Fischer C, et al. Multicentric validation of proteomic biomarkers in urine specific for diabetic nephropathy. PloS one. 2010;5:e13421.
Snell-Bergeon JK, Maahs DM, Ogden LG, Kinney GL, Hokanson JE, Schiffer E, et al. Evaluation of urinary biomarkers for coronary artery disease, diabetes, and diabetic kidney disease. Diabetes Technol Ther. 2009;11:1–9.
del Monte-Nieto G, Fischer JW, Gorski DJ, Harvey RP, Kovacic JC. Basic Biology of Extracellular Matrix in the Cardiovascular System, Part 1/4: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:2169–88.
LeBar K, Wang Z. Extracellular Matrix in Cardiac Tissue Mechanics and Physiology: Role of Collagen Accumulation. In: Madhurapantula, R. S., P.R.O., J. O., Loewy, Z., editors. Extracellular Matrix - Developments and Therapeutics [Internet]. London: IntechOpen; 2021 [cited 2022 May 13]. Available from: https://www.intechopen.com/chapters/75606 10.5772/intechopen.96585
Aihara K-I, Ikeda Y, Yagi S, Akaike M, Matsumoto T. Transforming growth factor-β1 as a common target molecule for development of cardiovascular diseases, renal insufficiency and metabolic syndrome. Cardiol Res. Pr. 2010;2011:175381.
Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-β signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206.
Bishop JE, Lindahl G. Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc Res. 1999;42:27–44.
Mels CM, Delles C, Louw R, Schutte AE. Central systolic pressure and a nonessential amino acid metabolomics profile: the African Prospective study on the Early Detection and Identification of Cardiovascular disease and Hypertension. J Hypertens. 2019;37:1157.
De Beer D, Mels CM, Schutte AE, Louw R, Delles C, Kruger R. Left ventricular mass and urinary metabolomics in young black and white adults: The African-PREDICT study. Nutr Metab Cardiovasc. 2020;30:2051–62.
Catapano AL, Pirillo A, Norata GD. Vascular inflammation and low-density lipoproteins: is cholesterol the link? A lesson from the clinical trials. Br J Pharm. 2017;174:3973–85.
Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70:111–3.
Bradley R. Gamma glutamyltransferase (GGT) as a biomarker of atherosclerosis. Biomarkers in cardiovascular disease. Dordrecht, Netherlands: Springer, 2016: p. 673-702.
Nystoriak MA, Bhatnagar A. Cardiovascular Effects and Benefits of Exercise. Front Cardiovasc Med. 2018;5:135.
Lubrano V, Balzan S. Role of oxidative stress-related biomarkers in heart failure: galectin 3, α1-antitrypsin and LOX-1: new therapeutic perspective. Mol Cell Biochem. 2020;464:143–52.
Argiles A, Siwy J, Duranton F, Gayrard N, Dakna M, Lundin U, et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. Plos one. 2013;8:e62837.
Acknowledgements
We are grateful to all individuals who voluntarily participated in this study. The dedication of the support and research staff as well as students at the Hypertension Research and Training Clinic at the North-West University is also duly acknowledged.
Funding
The research funded in this manuscript is part of an ongoing research project financially supported by the South African Medical Research Council (SAMRC) with funds from the National Treasury under its Economic Competitiveness and Support Package; the South African Research Chairs Initiative (SARChI) of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (GUN 86895); the SAMRC with funds received from the South African National Department of Health, GlaxoSmithKline R&D (Africa Non-Communicable Disease Open Lab grant), the UK Medical Research Council and with funds from the UK Government’s Newton Fund; and corporate social investment grants from Pfizer (South Africa), Boehringer-Ingelheim (South Africa), Novartis (South Africa), the Medi Clinic Hospital Group (South Africa) and kind contributions from Roche Diagnostics (South Africa). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors, and therefore, the NRF does not accept any liability in this regard.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HM is the co-founder and co-owner of Mosaiques Diagnostics. All other authors have no conflicts of interest to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Beer, D., Mels, C.M.C., Schutte, A.E. et al. A urinary peptidomics approach for early stages of cardiovascular disease risk: The African-PREDICT study. Hypertens Res 46, 485–494 (2023). https://doi.org/10.1038/s41440-022-01097-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01097-7
- Springer Nature Singapore Pte Ltd.