Abstract
Phytopathogenic bacteria are a major cause of crop mortality and yield reduction, especially in field cultivation. The lack of effective chemistry agri-bactericides is responsible for challenging field prevention and treatment, prompting the development of long-lasting solutions to prevent, reduce, or manage some of the most devastating plant diseases facing modern agriculture today and in the future. Therefore, there is an urgent need to find lead drugs preventing and treating phytopathogenic bacterial infection. Drug repurposing, a strategy used to identify novel uses for existing approved drugs outside of their original indication, takes less time and investment than Traditional R&D Strategies in the process of drug development. Based on this method, we conduct a screen of 700 chemically diverse and potentially safe drugs against Xanthomonas oryzae PV. oryzae ACCC 11602 (Xoo), Xanthomonas axonopodis PV. citri (Xac), and Pectobacterium atrosepticum ACCC 19901 (Pa). Furthermore, the structure-activity relationship and structural similarity analysis of active drugs classify potent agri-bactericides into 8 lead series: salicylanilides, cationic nitrogen-containing drugs, azole antifungals, N-containing group, hydroxyquinolines, piperazine, kinase inhibitor and miscellaneous groups. MIC values were evaluated as antibacterial activities in this study. Identifying highly active lead compounds from the screening of approved drugs and comparison with the currently applied plant pathogenic bactericide to validate the bactericidal activity of the best candidates and assess if selected molecules or scaffolds lead to develop new antibacterial agents in the future. In conclusion, this study provides a possibility for the development of potent and highly selective agri-bactericides leads.
Similar content being viewed by others
Introduction
The development of human agricultural civilization has a history of nearly 10,000 years [1]. However, a sharply increased demand in food production has attracted unprecedented attention as the growing world population makes it an important responsibility to protect crops from phytopathogenic bacteria [2,3,4]. Phytopathogenic bacteria cause enormous yield loss in various crops worldwide every year, particularly Xanthomonas oryzae PV. Oryzae (Xoo), Xanthomonas axonopodis PV. citri (Xac), and Pectobacterium atrosepticum (Pa) [5,6,27,28]. In addition, in the process of agribactericides discovery, the number of screening new active compounds increased significantly. Searching for active lead compounds from approved drugs and then carrying out structural modification or derivatization has been proved to be a successful way to find agribactericides with new action modes [29,30,31]. However, the discovery of lead compound remains a major challenge, The number of compounds rose from 52,500 in 1995 to 140,000 in 2005 to discover a new agrochemical lead compound [16, 32]. Thus, the lead compound is a prerequisite for the discovery of agrochemicals.
To this extent, we screened 700 approved drugs against Xoo, Xac and Pa. Among them, the structure-activity relationship and structural similarity analysis of active drugs classify potent agri-bactericides into 8 lead series: salicylanilides, cationic nitrogen-containing drugs, azole antifungals, N-containing group, hydroxyquinolines, piperazine, kinase inhibitor, and miscellaneous groups.
Materials and methods
Bacterial Strains and growth conditions
Xoo ACCC 11602 and Pa ACCC 19901 were purchased from the Agricultural Culture Collection of China (ACCC). Xac was provided by Professor Song Yang’s research group from Guizhou University. The bacteria were experienced the 16 S ribosome gene series alignment, the comparison results are provided in the Supporting Information.
The above strains containing 30% glycerol were frozen at – 80 °C in the laboratory. The frozen strains were taken out, scribed on nutrient broth (NB) solid media, culturing at 28 °C until a single colony grew. Then, a single colony was picked from the solid media to the nutrient broth (NB) media and cultured to the logarithmic growth phase at 28 °C on a shaker incubator at 180 rotations per min (rpm). The strain in the logarithmic growth phase was diluted with nutrient broth (NB) media to about 106 CFU ml−1 for later use.
The nutrient broth (NB) media: 3.0 g of beef extract, 5.0 g of peptone, 1.0 g of yeast extract, 10.0 g of sucrose, 8.0 g of sodium chloride, 1 L of distilled water, pH = 7.0 − 7.2.
Chemicals and compounds
All drugs or compounds were purchased from commercial suppliers and available without purification (unless stated otherwise). The above-tested drugs were dissolved in DMSO at concentrations of 100,000 μg ml−1 and stored at −4 °C or −20 °C. Then, to a 2 ml tube, 998 μl of nutrient broth (NB) media, 2 μl of the compounds dissolved in DMSO were added so that the final concentration is 200 μg ml−1 for later use.
In vitro antibacterial assay
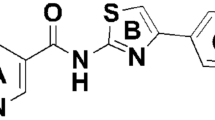
Antibacterial activities of target drugs and compounds were tested against three phytopathogenic bacteria (Xoo, Xac, and Pa) using the turbidimetric method [3 and Fig. 4, the first azole series we investigated was 1-(phenylethyl)imidazole derivatives (lead series 6), active drugs of this lead series contained fenticonazole nitrate, miconazole, econazole, butoconazole nitrate (MIC90 ranged from 3.12 to 12.5 μg ml−1). The preliminary structure-activity relationships indicated that the substitution of benzyl contributed to increaseing the antibacterial activity, introduction of oxygen and sulfur atoms to form ethers could cause a more potent antibacterial effect. Among the 1-(phenylethyl)imidazole derivatives, the position of the halogen substituent on the benzene ring seemed to greatly improve the antibacterial activity, especially with 2,6-dichloro-substituted. Miconazole and econazole, a broad-spectrum imidazole fungicide, inhibit synthesis in fungal cell membranes and RNA, the screening and further confirmation revealed that miconazole and econazole were found to exhibit a considerable activity against Xoo (MIC90 = 12.5 μg ml−1). Nitroimidazoles and benzimidazoles are our second lead series of azoles (lead series 7), with triclabendazole being the standout for antibacterial activity(the MIC value was 6.25 μg ml−1 against Xoo and Xac). Interestingly, our screening identified the third azole series (lead series 8), simple-structured thiazolinones exhibit strong antibacterial activity. The substituent of thiazolinone affects the activity of drugs, methyl and chlorine decreased the activity 4-time (5-chloro-2-methylisothiazol-3(2H)-one), as compared to the unsubstituted thiazol-3-one. In addition, the introduction of a long chain into the nitrogen atom of thiazolinone does not indicate an increase or decrease in activity compared with thiazol-3-one. Therefore, nitrogen may not be the key factor affecting the anti-agribacterial activity. The benzothiazoles, 1,2-benzisothiazol-3(2H)-one, 2-methyl-1,2-benzothiazol-3(2H)-one, and 6-fluoro-1,2-benzisothiazol-3(2H)-one, show considerable activities, especially 6-fluoro-1,2-benzisothiazol-3(2H)-one with the substitution of fluorine on its phenyl rings, which may be accountable for the higher activity as a functional group.
Hydroxyquinolines
Hydroxyquinolines are established to own wealthy biological activities and can be used as herbicides, disinfectants, preservatives, chemical intermediates, etc, that determines their wide application within the field of medication. Our research group previously conducted research on 8-hydroxyquinoline as metal chelators against agricultural fungi, and the results showed that this kind of compounds has excellent antifungal activity, revealing great potential as agricultural fungicides [41].
As shown in Table 4 and Fig. 5, the results of the screening experiments indicated that the quinoline derivatives with 8-hydroxyl group exhibited increased antibacterial activity at primary screening of 100 μg ml−1, compared with other positions of hydroxyl substitution, such as the 2, 5, 6 hydroxyl groups. With 8-hydroxyquinoline (lead series 9) as the skeleton, different group substitutions even have different antibacterial effects. When the NO2 on the 5-position is substituted, the activity of 8-hydroxyquinoline against three pathogenic bacteria is greatly improved, with the increased bactericide result against Xoo, Xac, Pa by 4, 16 and 8 times respectively (the MICs are 0.39, 6.25, 1.56). Substitution of Cl and Br at the 5-position produces a similar effect either. However, CH3 substitution did not appear to have a positive effect, even reduced activity against Xoo. In addition, 8-hydroxyquinoline bears two groups substituents at the 5 and 7 positions have less potential, especially 5,7-dibromo-8-hydroxyquinoline. Studies have shown that the ability of the 8-hydroxyquinoline scaffold to chelate divalent ions make this molecule an important fragment to interact with metalloproteins in microorganisms as targets, which may be the main reason for its antibacterial activities.
It is worth mentioning that the commercialized chloroquinadol, as one of the main components of the clinically used drug Ke**gbao, is well known for its anti-Candida albicans effect. In fact, our experiments show that its in vitro antibacterial activity against Xoo is even better than that against Candida albicans, with MIC of 0.39 μg ml−1 against Xoo and 0.12 μg ml−1 against Ca (The data were measured by us simultaneously). Overall, our repurposing of the commercially available drugs, 8-hydroxyquinoline, endows it with a broader application, is warrant further investigation within the area of controling phytopathogenic pathogens.
N-containing group
As shown in Table 5 and Fig. 6, N-containing group drugs were screened as lead series 10. The pharmacophore of these compounds includes amines, ureas and guanidines. Amines are nitrogenous aliphatic or heterocyclic substances with biological functions. **njunan is a precursor in the synthesis of Junduqing, a broad-spectrum bactericide which was successfully developed by China Shandong Chemical Development Center in 1989. It has been used for various crops to control agricultural diseases caused by fungi, bacteria and viruses for many years. **njunan has good antibacterial activity against three phytopathogenic bacteria in this screening experiment. In order to investigate the impact of amino groups on antibacterial activity, the activity of commercial fatty amines was tested, but these fatty amines have no antibacterial activity. which shows that the exposed amino group is not the active center. **njunan to a reasonable improvement of activity only when the bilateral amino groups are connected by a long aliphatic chain. In addition, compounds with urea and guanidine groups such as triclocarban and chlorhexidine acetate have been widely used in the field of medical sterilization and disinfection, which have broad-spectrum antimicrobial activity and are harmless in direct contact with the human skin. These N-containing groups as the hydrophilic head of these molecules contain strong positive charges and adsorb negatively charged bacterial cell membranes by electrostatic interaction. Our results suggest that these drugs have equal effect against plant bacteria.
Piperazine
As shown in Table 6 and Fig. 7, the category discussion of lead series 11 was based on the presence of a central heterocyclic ring system containing at least one nitrogen atom (piperazine and piperidine groups). From the structure-activity point of view, the nature and position of the electron donating functional groups on the piperazine and piperidine core may contribute to the antibacterial activity. It is worth mentioning that Penfluridol, a commercial long-acting antipsychotic indicated for the maintenance treatment of chronic schizophrenia, has high antibacterial activity against Xoo and Xac with the MICs of 3.12 μg ml−1, providing a basis again for the strategy of drug-repurposing.
Kinase inhibitors
Kinase inhibitors attracted much attention for a long time, owing to their significant role in the field of anti-tumor. However, bacterial growth processes are also affected by signal pathways. Hence, many studies have focused on the application of kinase inhibitors to the antibacterial field recently. For instance, Philipp Le found the anti-cancer drug Sorafenib showed high anti-bacterial activity against MRSA strains and did not induce in vitro resistance.
As shown in Table 7 and Fig. 8, among this established series (lead series 12), 4,4' -(dithiodicarbonothioyl)dimorpholine (JX06) is well known as a PDK inhibitor, which usually binds covalently to cysteine residues in an irreversible manner resulting in antitumor activity. In this study, we screened the 53 key kinase inhibitors led to the discovery of JX06 as a outstanding hit effectively killing the two specific plant pathogenic strains at concentrations of micromoles per milliliter. The MICs of JX06 were 6.25 and 12.5 μg ml−1 against Xoo and Xac respectively. Besides, Perifosine also has a similar effect (the MICs of 6.25 and 25 μg ml−1 against Xoo and Xac respectively), which may be the result of the combined effect of cation membranes permeability and certain signaling pathway regulation. Taken together, these results support the potential application of these kinase inhibitors with antibacterial activity for bacterial disease control in plants.
Miscellaneous groups
As shown in Table 8 and Fig. 9, the last series (lead series 13) is some chemically dispersed drugs. Drugs which are conducted in this screen category are quinine, sulfa anti-inflammatory, nucleoside anticancer, cephalosporin antimicrobial and S-containing drugs which include thioether, mercaptan, disulfide drugs. Highly active anti-agribacterial drugs identified in the screening are listed by class. It was also attracted that the derivative of pyrithione (Zinc pyrithione, Sodium omadine, Copper pyritione, and Pyrion disulfide) has reasonable anti-phytopathogenic bacteria activity. In previous reports, Zinc pyrithione passed the increase in cellular zinc levels, decrease in lipase expression, and inhibition of mitochondrial function against M. restricta[42]; Bithionol exhibits bactericidal activity against both antibiotic-resistant S. aureus with its ability to pass through and embed in bacterial membranes lipid bilayers [43]. The anti-phytopathogenic bacteria activities of double phenol-containing drugs (Dichlorophen, Triclosan, and Bithionol) might be attributed to the anti-corrosion and weak acidity of the phenolic part. Among them, triclosan and dichlorophen have the strongest antibacterial activity, both drugs contain similar structure, the MIC90 ranged from 3.12 to 25 μg ml−1; Abafungin was found to have potentiality antifungal activity whether the pathogens are growing or resting [44]. The anti-phytopathogenic bacteria activity of these five drugs against Xoo, Xac, and Pa has never been reported and therefore is worth further exploration; Pleuromutilin is a broad-spectrum diterpene antibiotic produced by Pleurotus mutilus. It inhibits bacterial growth by disturbing bacterial protein synthesis. Retapamulin and valnemulin hydrochloride are based on pleuromutilin antibiotics. In this study, retapamulin and valnemulin show excellent anti-phytopathogenic bacterial activities against Xoo with a MIC of 6.25 and 0.78 μg ml−1. Although there is no necessary connection between the activity and structure of this group of drugs, these results provide a approach based structure screening for the repurposing of commercially available drugs, expecting to quicken the discovery of drugs against phytopathogenic bacteria.
Risk
Although drug repurposing provides a rapid and efficient method to screen antibacterial leads from approved drugs, which are making a significant impact on the development of antimicrobial resistance (AMR) [45, 46]. When clinical drugs or other drugs are used as agrichemicals, it may provide new resistant strains and accelerate the development of AMR. The clinical drugs or other antimicrobial agents use in agriculture practice, particularly as agrichemicals used in the field, are one of the causes of the development of AMR. However, this risk is extending to humans through the food chain, the use of antimicrobial agents in food and agriculture has a direct or indirect impact on the development of antimicrobial resistance (AMR) in plant-associated bacteria [47]. For those reasons, alternative antimicrobials are also needed to combat the phenomenon of AMR in clinical settings and agricultural practices, such as in farms and food premises. To reduce or replace the use of common antibiotics, drug repurposing provides new lead compounds from the antibacterial screening of approved drugs, while also paying attention to the risks that exist in drug repurposing.
Conclusion
Our work provides a basis for drug discovery that enables the discovery of agricultural bacterial drugs superior to current traditional methods. Hopefully, we will enable the development of repurposed approved drugs to be effective against phytopathogenic bacteria. In addition to drug repurposing, approved drugs-with known well-documented safety, stability, and toxicological effects-can be used as new lead compounds.
References
Doebley JF, Gaut BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127:1309–21.
Oerke EC, Dehne HW. Safeguarding production—losses in major crops and the role of crop protection. Crop Prot. 2004;23:275–85.
Atkinson D, Litterick AM, Walker KC, Walker R, Watson CA. Crop protection–what will shape the future picture? Pest Manag Sci. 2004;60:105–12.
Quintana-Rodriguez E, Duran-Flores D, Heil M, Camacho-Coronel X. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci horticulturae. 2018;237:207–20.
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, et al. Top 10 plant pathogenic bacteria in molecular plant pathology: Top 10 plant pathogenic bacteria. Mol plant Pathol. 2012;13:614–29.
Mattinen L, Nissinen R, Riipi T, Kalkkinen N, Pirhonen M. Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum. Proteom (Weinh). 2007;7:3527–37.
Ke Y, Wu M, Zhang Q, Li X, **ao J, Wang S. Hd3a and OsFD1 negatively regulate rice resistance to Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola. Biochemical biophysical Res Commun. 2019;513:775–80.
Cernadas RAS, Camillo LR, Benedetti CE. Transcriptional analysis of the sweet orange interaction with the citrus canker pathogens Xanthomonas axonopodis pv. citri and Xanthomonas axonopodis pv. aurantifolii. Mol plant Pathol. 2008;9:609–31.
Seck PA, Diagne A, Mohanty S, Wopereis MCS. Crops that feed the world 7: Rice. Food Security. 2012;4:7–24.
Li C, Zhang J, Ren Z, **e R, Yin C, Ma W, et al. Development of ‘multiresistance rice’ by an assembly of herbicide, insect and disease resistance genes with a transgene stacking system. Pest Manag Sci. 2021;77:1536–47.
Le Dang Q, Shin TS, Park MS, Choi YH, Choi GJ, Jang KS, et al. Antimicrobial activities of novel mannosyl lipids isolated from the biocontrol fungus Simplicillium lamellicola BCP against phytopathogenic bacteria. J Agric Food Chem. 2014;62:3363–70.
**ang M, Song YL, Ji J, Zhou X, Liu LW, Wang PY, et al. Synthesis of novel 18beta-glycyrrhetinic piperazine amides displaying significant in vitro and in vivo antibacterial activities against intractable plant bacterial diseases. Pest Manag Sci. 2020;76:2959–71.
Wang P, Gao M, Zhou L, Wu Z, Hu D, Hu J, et al. Synthesis and antibacterial activity of pyridinium-tailored aromatic amphiphiles. Bioorg Med Chem Lett. 2016;26:1136–9.
Pan X, Xu S, Wu J, Luo J, Duan Y, Wang J, et al. Screening and characterization of Xanthomonas oryzae pv. oryzae strains with resistance to pheazine-1-carboxylic acid. Pestic Biochem Physiol. 2018;145:8–14.
Dai X, Zhao Y, Li J, Li S, Lei R, Chen X, et al. Thiazolium-derivative functionalized silver nanocomposites for suppressing bacterial resistance and eradicating biofilms. N. J Chem. 2018;42:1316–25.
Hao G-F, Yang S-G, Huang W, Wang L, Shen Y-Q, Tu W-L et al. Rational design of highly potent and slow-binding cytochrome bc(1) inhibitor as fungicide by computational substitution optimization. Sci Rep. 2015;5:1–10.
Lamberth C, Jeanmart S, Luksch T, Plant A. Current Challenges and Trends in the Discovery of Agrochemicals. Sci (Am Assoc Advancement Sci). 2013;341:742–6.
Demarque DP, Espindola LS. Challenges, advances and opportunities in exploring natural products to control arboviral disease vectors. Front Chem. 2021;9:1–10.
Sparks TC, Lorsbach BA. Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag Sci. 2017;73:672–7.
Sparks TC, Wessels FJ, Lorsbach BA, Nugent BM, Watson GB. The new age of insecticide discovery-the crop protection industry and the impact of natural products. Pestic Biochem Physiol. 2019;161:12–22.
Torres NS, Abercrombie JJ, Srinivasan A, Lopez-Ribot JL, Ramasubramanian AK, Leung KP. Screening a commercial library of pharmacologically active small molecules against Staphylococcus aureus biofilms. Antimicrobial agents Chemother. 2016;60:5663–72.
Wilkinson GF, Pritchard K. In vitro screening for drug repositioning. 20. Los Angeles, CA: SAGE Publications; 2015. p. 167–79.
Dudley JT, Deshpande T, Butte AJ. Exploiting drug-disease relationships for computational drug repositioning. Brief Bioinforma. 2011;12:303–11.
Kaiser M, Maser P, Tadoori LP, Ioset J-R, Brun R. Antiprotozoal activity profiling of approved drugs: a starting point toward drug repositioning. PloS One. 2015;10:e0135556–e0135556.
Novac N. Challenges and opportunities of drug repositioning. Trends Pharmacol Sci (Regul ed). 2013;34:267–72.
Oliveira IM, Borges A, Simões M The potential of drug repurposing to face bacterial and fungal biofilm infections. Recent Trends in Biofilm Science and Technology, 2020, pp 307-28.
Ashburn TT, Thor KB. Drug repositioning: identifying and develo** new uses for existing drugs. Nat Rev Drug Disco. 2004;3:673–83.
Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–68.
Gu J, Gui Y, Chen L, Yuan G, Lu HZ, Xu X. Use of natural products as chemical library for drug discovery and network pharmacology. PloS One 2013;8:e6283.
Ohlson S, Duong-Thi M-D. Fragment screening for drug leads by weak affinity chromatography (WAC-MS). Methods (San Diego, Calif). 2018;146:26–38.
Nosengo N. New tricks for old drugs. Nat (Lond). 2016;534:314–6.
Delaney J, Clarke E, Hughes D, Rice M. Modern agrochemical research: a missed opportunity for drug discovery? Drug Discov today. 2006;11:839–45.
Li P, Hu D, **e D, Chen J, ** L, Song B. Design, Synthesis, and Evaluation of New Sulfone Derivatives Containing a 1,3,4-Oxadiazole Moiety as Active Antibacterial Agents. J Agric Food Chem. 2018;66:3093–3100.
Fan Z, Shi J, Luo N, Ding M, Bao X. Synthesis, crystal structure, and agricultural antimicrobial evaluation of novel quinazoline thioether derivatives incorporating the 1,2,4-triazolo[4,3-a]pyridine moiety. J Agric Food Chem. 2019;67:11598–606.
Guo T, **a R, Chen M, He J, Su S, Liu L, et al. Biological activity evaluation and action mechanism of chalcone derivatives containing thiophene sulfonate. RSC Adv. 2019;9:24942–50.
Luo HZ, Guan Y, Yang R, Qian GL, Yang XH, Wang JS, et al. Growth inhibition and metabolomic analysis of Xanthomonas oryzae pv. oryzae treated with resveratrol. BMC Microbiol. 2020;20:117.
Chen J, Wang X, Han H. A new function of graphene oxide emerges: inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. J Nanoparticle Res. 2013;15:1–14.
Wu S, Shi J, Chen J, Hu D, Zang L, Song B. Synthesis, Antibacterial Activity, and Mechanisms of Novel 6-Sulfonyl-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole Derivatives. J Agric Food Chem. 2021;69:4645–54.
Lefevere H, Bauters L, Gheysen G. Salicylic acid biosynthesis in plants. Front Plant Sci. 2020;11:1–7.
Kratky M, Stepankova S, Houngbedji N-H, Vosatka R, Vorcakova K, Vinsova J. 2-Hydroxy-N-phenylbenzamides and Their Esters Inhibit Acetylcholinesterase and Butyrylcholinesterase. Biomolecules (Basel, Switzerland). 2019;9:698–714.
Yin X-D, Sun Y, Lawoe RK, Yang G-Z, Liu Y-Q, Shang X-F, et al. Synthesis and anti-phytopathogenic activity of 8-hydroxyquinoline derivatives. RSC Adv. 2019;9:387–99.
Park M, Cho Y-J, Lee YW, Jung WH. Understanding the mechanism of action of the anti-dandruff agent zinc pyrithione against Malassezia restricta. Sci Rep. 2018;8:12086–97.
Kim W, Zou G, Hari TPA, Wilt IK, Zhu W, Galle N, et al. A selective membrane-targeting repurposed antibiotic with activity against persistent methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci. 2019;116:16529–34.
Borelli C, Schaller M, Niewerth M, Nocker K, Baasner B, Berg D, et al. Modes of action of the new arylguanidine abafungin beyond interference with ergosterol biosynthesis and in vitro activity against medically important fungi. Chemother (Basel). 2008;54:245–59.
Srivastava J, Chandra H, Nautiyal AR, Kalra SJS. Antimicrobial resistance (AMR) and plant-derived antimicrobials (PDA(m)s) as an alternative drug line to control infections. 3 Biotech. 2014;4:451–60.
Cheng G, Ning J, Ahmed S, Huang J, Ullah R, An B et al. Selection and dissemination of antimicrobial resistance in Agri-food production. Antimicrobial Resistance Infection Control. 2019;8:158–71.
Compri M, Mader R, Mazzolini E, de Angelis G, Mutters NT, Rajendran NB, et al. White Paper: Bridging the gap between surveillance data and antimicrobial stewardship in the animal sector-practical guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net networks. J Antimicrobial Chemother. 2020;75:52–66.
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (22177043, 21877056) and The Natural Science Foundation of Gansu Province (20JR5RA311); Support was also supplied by the Key Program for international S&T cooperation projects of China Gansu Province (18YF1WA115).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Wang, YR., He, YH. et al. Drug repurposing strategy part 1: from approved drugs to agri-bactericides leads. J Antibiot 76, 27–51 (2023). https://doi.org/10.1038/s41429-022-00574-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-022-00574-y
- Springer Japan KK