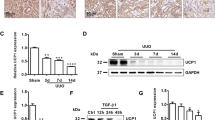
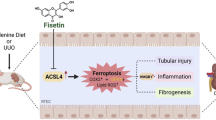
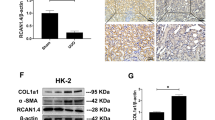
Abstract
Kidney fibrosis is considered to be the ultimate aggregation pathway of chronic kidney disease (CKD), but its underlying mechanism remains elusive. Protein kinase C-delta (PKC-δ) plays critical roles in the control of growth, differentiation, and apoptosis. In this study, we found that PKC-δ was highly upregulated in human biopsy samples and mouse kidneys with fibrosis. Rottlerin, a PKC-δ inhibitor, alleviated unilateral ureteral ligation (UUO)-induced kidney fibrosis, inflammation, VDAC1 expression, and cGAS-STING signaling pathway activation. Adeno-associated virus 9 (AAV9)-mediated VDAC1 silencing or VBIT-12, a VDAC1 inhibitor, attenuated renal injury, inflammation, and activation of cGAS-STING signaling pathway in UUO mouse model. Genetic and pharmacologic inhibition of STING relieved renal fibrosis and inflammation in UUO mice. In vitro, hypoxia resulted in PKC-δ phosphorylation, VDAC1 oligomerization, and activation of cGAS-STING signaling pathway in HK-2 cells. Inhibition of PKC-δ, VDAC1 or STING alleviated hypoxia-induced fibrotic and inflammatory responses in HK-2 cells, respectively. Mechanistically, PKC-δ activation induced mitochondrial membrane VDAC1 oligomerization via direct binding VDAC1, followed by the mitochondrial DNA (mtDNA) release into the cytoplasm, and subsequent activated cGAS-STING signaling pathway, which contributed to the inflammation leading to fibrosis. In conclusion, this study has indicated for the first time that PKC-δ is an important regulator in kidney fibrosis by promoting cGAS-STING signaling pathway which mediated by VDAC1. PKC-δ may be useful for treating renal fibrosis and subsequent CKD.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) remains a worldwide public health concern affecting 8–15% of the global population [1]. Renal tubulointerstitial fibrosis is widely thought to be the common pathway that leads to end-stage renal failure in almost all progressive CKD, and it is also a crucial pathological manifestation of end-stage renal disease [2]. Kidney fibrosis is characterized by excessive deposition of extracellular matrix proteins such as fibronectin and collagens, and increased generation of α-smooth muscle actin (α-SMA) in the interstitium [3]. Collecting evidence demonstrated that the infiltration of inflammatory cells, and accompanied by increased expression of monocyte chemoattractant protein-1(MCP-1), interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) are involved in the renal fibrosis process [4]. However, the underlying mechanism of tubulointerstitial fibrosis remain obscure, and the treatment is lacking.
Protein Kinase C (PKC) is a family of serine/threonine kinases with several subtypes, including traditional PKCs (α, β, γ), novel PKCs (δ, ε, θ, η), and atypical PKCs (ζ, λ/ɩ) [5, 6]. PKC-δ also called as PRKCD, which has the ability to regulate cell growth, differentiation, apoptosis, transformation and tumorigenicity [7]. Previous study showed that cardiac ischemia and reperfusion led to the translocation of PKC-δ to mitochondria then affected the activity of downstream apoptotic factors through the release of cytochrome C [8]. PRKCD is localized to mitochondria and regulates recruitment of ULK1-ATG13 upon induction of mitophagy [9, 10]. In addition, PKC-δ inhibition protected against cisplatin nephrotoxicity by upregulating autophagy [Mice model Mouse unilateral ureteral obstruction (UUO) model was established as described following. The hair was removed from the back of the mice after anesthetized by inhalation of 4% isoflurane. The mice were placed in the prone position on the operating table and the surgical area was sterilized. An oblique incision was made in the back of the mouse, parallel to the costal arch, exposed the inferior renal pole and the upper ureter, and the left ureter was ligated with No. 6 silk thread. Wild-type (WT) littermates which underwent a sham operation were used as control subjects. Sham mice and/or UUO mice were treated with Rottlerin (10 mg/kg), C-176 (750 nmol per mouse), or VBIT-12 (20 mg/kg) (Supplementary Table S2) daily by intraperitoneal injection. Ischemia/reperfusion (I/R)-induced AKI mouse model was performed by exposing bilateral kidneys. Kidney ischemia was performed for 30 min by clam** the renal pedicle through a lateral ventral incision, the arterial clips were released for reperfusion. The sham mice underwent the same procedure except that the kidney pedicle was not clamped. The mice were housed in a specific pathogen free facility. For all studies, mice were housed under controlled environmental conditions under a 12-h light/dark cycle and allowed ad libitum access to rodent chow and water. The 8-week-old age C57BL/6J wild type (WT) male mice were treated with adeno-associated virus 9 carrying the shVDAC1 for interference of VDAC1 gene. Adeno-associated virus 9 vectors (AAV9) carrying VDAC1 interference sequences (AAV-shVDAC1-EGFP, 1.3*1012 vg/ml) and its negative control (AAV-Control-EGFP) were designed by Hanheng Biotechnology Company (Shanghai, China). Each mouse was injected with 100 ul 1.3*1012 vg/ml AAV9 carrying the shVDAC1 (AAV-shVDAC1-EGFP) or negative control (AAV-EGFP) through tail. UUO or Sham operation was performed at 3 weeks after adeno-associated virus injection. Renal biopsy residual samples were recruited in the Second Hospital of Hebei Medical University. According to the principles of the Declaration of Helsinki, all human studies were conducted with informed consent of patients and approved by the Medical Ethics Committee of Hebei Medical University (No. 2021033). In this study, twenty five renal biopsy samples from adults were obtained. Including 5 normal renal tissue samples from the distal kidney after local tumor resection, 5 patients with type 2 diabetes mellitus with nephropathy, 5 patients with IgA nephropathy, 5 patients with focal segmental glomerular sclerosis and 5 patients with Lupus nephritis V. Mouse kidney tissues were fixed in 4% paraformaldehyde for 48 h, dehydrated with gradient alcohol, embedded in paraffin, and then sectioned into slices about 3 μm, baked and removed wax. Haematoxylin Eosin (H&E), Sirius Red (Supplementary Table S2), and Masson’s trichrome staining were performed according to a standard protocol. Damaged tubular cells were evaluated as described previously [65]. Lesions were graded on a scale from 0 to 4: 0, normal; 1, <25% damage; 2, 25–50% damage; 3, 50–75% damage; 4, >75% damage. Immunohistochemical staining was performed with SP kit according to the instruction. The paraffin section was repaired with Tris-EDTA (PH = 9.0) repair solution for 8 min, then treated with endogenous peroxidase blockers, and incubated goat serum about 30 min at 37 °C. The kidney sections were incubated with primary antibodies overnight at 4 °C, followed by incubation with appropriate amount of enzyme-labeled sheep anti-mouse/Rabbit IgG polymer (Zhongshan **qiao Biotechnology, Bei**g, China) for 40 min at 37 °C, and detected with DAB kit (Zhongshan**qiao Biotechnology, Bei**g, China) for 1-2 min. Olympus microscope (Olympus, BX71, Tokyo, Japan) was used to capture the images. Quantitative analysis of positive staining was used by ImageJ software (NIH). The human tubular cell line HK-2 (purchased from China Centre for Type Culture Collection) was cultured in DMEM-F12 (1:1) medium (Gibco, USA) supplemented with 100 U/mL penicillin (Gibco, USA) and 10% fetal bovine serum (CellMax, China) in 5% CO2 and 20.9% O2 air at 37 °C. Cells attained 70–80% convergence in normal conditions (5% CO2 and 20.9% O2) and then grew in a medium without serum under hypoxic conditions (5% CO2, and 1% O2). HK-2 cells were transfected with siSTING and disrupted RNA (Negative control, NC) using transfection reagent (GenePharma, Shanghai, China) according to the manufacture’s protocols. And HK-2 cells were treated with C-176 (3 μM), Rottlerin (1 μM), VBIT-12 (20 μM) or dideoxycytidine (ddC, 40 μg/ml) (Supplementary Table S2)respectively under normoxia or hypoxia conditions. Total proteins from HK-2 cells and renal tissues were extracted using the radio immunoprecipitation assay (RIPA) lysis buffer (BestBio, Shanghai, China). Nuclear protein and cytoplasma protein were extracted using Nuc-Cyto-Mem Preparation Kit (Applygen Biotechnology, Bei**g, China). The protein concentration was measured using the BCA protein kit (Solarbio, Bei**g, China). Equal amounts of protein were separated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, MA, USA). And then, membranes were incubated with primary antibodies overnight at 4 °C (Supplementary Table S1). Image acquisition was performed using Amersham Imager 600 (General Electric Company, USA) or Tanon 4800 (Bei**g, China). Band densitometry was analyzed using ImageJ software (NIH). Total RNA was obtained by TRIzol reagent (TIANGEN, Bei**g, China) from HK-2 cells and kidney tissues, and cDNA was prepared using MonScript™ RTIII (Monad, Guangdong, China) according to the instructions. The primers used were listed in Supplementary Table 3. Real-time PCR was performed in a 96-well optical reaction plate using MonAmpTM ChemoHS qPCR Mix (Monad, China). Quantitative PCR reactions were performed on Agilent Mx3000P QPCR Systems (Agilent, CA, USA). The experiment was repeated three times, and the relative gene expression levels were calculated using the 2−ΔΔCt method. Mitochondrial DNA (mtDNA) in the cytosolic fraction was isolated from HK-2 cells as described previously [27, 66]. HK-2 cells were lysed by mild detergent (0.1% NP-40) and incubated on ice for 15 min. Lysates were centrifuged at 13,000 rpm for 15 min at 4 °C. Cytosolic mtDNA from supernatant cytosolic fraction was purified using a DNeasy Blood & Tissue Kit (QIAGEN) according to manufacturer’s instructions. Quantitative PCR was employed to measure mtDNA using SYBR Premix Ex TaqTM II (Takara, Japan) on Agilent Mx3000P QPCR Systems (Agilent, CA, USA). The data were normalized and analyzed using the 2−ΔΔCt method. Primer sequences for mtDNA determination were shown in Supplementary Table 3. HK-2 cells were grown and stimulated on a slide in a six-well plate, washed with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. Cells were treated with 0.3% Triton X-100/PBS about 8–10 min at 37 °C, then incubated goat serum about 30 min at 37 °C. Paraffin-embedded sections were dewaxed, hydrated and repaired with Tris-EDTA (PH = 9.0). HK-2 cells and sections were incubated with primary antibodies overnight at 4 °C. Then the FITC conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Crux, CA) was incubated for 2 h at 37 °C. The nucleus was stained with DAPI (SouthernBiotech, America) and the stained slides were imaged using a laser confocal microscope (SP8, Leica, Germany). Frozen sections of kidney tissue (6 um) were fixed with cold acetone for 15 min and washed with PBS, incubated goat serum about 30 min at 37 °C. The sections were incubated with primary antibodies for PKC-δ, AQP1, calbindin-D28k, and VDAC1 in PBS overnight at 4 °C. HK-2 cells were grown and stimulated on a slide in a six-well plate, washed with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. Treatment with 0.3% Triton X-100/PBS about 8–10 min at 37 °C, then incubated with goat serum about 30 min at 37 °C. The slides were incubated with primary antibodies for PKC-δ, and VDAC1 in PBS at 4 °C overnight. Then the cells or sections were incubated with goat anti-rabbit/mouse IgG conjured to Alexa Fluor 488/594 (Abcam, Cambridge, MA, USA) for 2 h at 37 °C and the nucleus was stained using DAPI. The stained samples were viewed using a confocal microscope (SP8, Leica, Germany). HK-2 cells were stained with MitoTracker™ Red CMXRos (200 nM, Thermo Fisher Scientific, US) at 37 °C for 30 min, washed with PBS and fixed with cold 4% paraformaldehyde for 30 min, permeated with 0.3% Triton X-100 for 8 min at 37 °C. Goat serum was incubated at 37 °C for 30 min, and then incubated with anti-PKC-δ primary antibody in a wet chamber at 4 °C overnight. Then the FITC conjugated goat anti-rabbit IgG (1:100; Santa Cruz Biotechnology, Santa Crux, CA) was incubated for 2 h at 37 °C before DAPI staining. The stained samples were viewed using a confocal microscope (SP8, Leica, Germany). To evaluated the mitochondrial morphology, the average length of mitochondria was measured after MitoTracker Red staining [67]. Mitochondrial reactive oxygen species (mtROS) was detected by MitoSox Red (Thermo Fisher Scientific, US) according to the manufacturer’s instructions. HK-2 cells were incubated with MitoSox Red at a final concentration of 5 μM at 37 °C for 30 min. The stained cells washed three times with Hank’s balanced salt solution (HBSS) and observed using a confocal microscope (Leica, Germany). The mitochondrial ROS intensity was quantified using the software Image-Pro Plus 6.0. Mitochondrial membrane potential was detected with the JC-1 kit (C2006, Beyotime, China) according to the manufacturer’s instructions. In brief, added 1 ml JC-1 working fluid to the holes of each six-well plate, incubated at 37 °C for 30 min, and washed with JC-1 liquid buffer in a dark environment for 3 times. 2 ml cell culture medium was added. The stained samples were viewed using a confocal microscope (Leica, Germany). The MMP values were expressed as the ratio of red to green fluorescence levels. HK-2 cells were washed with ice-cold PBS and lysed with RIPA buffer. After centrifugation at 12000 rpm for 30 minutes at 4 °C, the protein A-Agarose (Santa Cruz, Dallas, TX) were incubated with anti-PKC-δ (1909 mg/mL) or anti-VDAC1 (1000 μg/ml) with continuous shaking for 8 h at 4 °C. After washing the IP complexes, the beads were boiled with 1× buffer for 7 min, and then using immunoblot analysis. Cells were treated with the hypoxia as above, collected, washed with PBS, pH 8.3, and then were incubated with EGS (100 mM, 40 min, 30 °C). To remove excess crosslinker, 1.5 M Tris HCl (pH 7.8) was added to a final concentration of 20 mM, incubated at room temperature for 5 min, and then centrifuged at 10000 × g for 5 min. The samples were analyzed by SDS-PAGE with anti-VDAC1. Data are expressed as Mean ± standard error (SEM). The results from at least three independent experiments. Statistical analysis was performed using one-way (ANOVA) analysis of variance. P < 0.05 was defined as statistically significant.Adeno-associated viruses delivery
Clinical specimens
Histology and immunohistochemical (IHC) staining
Cell culture and treatment
Western blot
Quantitative real-time PCR (qPCR)
Mitochondrial DNA release assay
Immunofluorescence staining
Immunofluorescence double staining
MitoTracker Red and immunofluorescence staining
Measurement of mtROS
Mitochondrial transmembrane potential (MMP)
Coimmunoprecipitation assay
VDAC cross-linking assay
Statistics
Data availability
The original data for Western Blot, as well as the prime sequence of genes are available in the supplementary materials.
References
Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019;65:16–36.
Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K. Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol. 2016;12:426–39.
Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–38.
Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15:144–58.
Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–12.
Newton AC. Protein kinase C: perfectly balanced. Crit Rev Biochem Mol Biol. 2018;53:208–30.
Duquesnes N, Lezoualc’h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers? J Mol Cell Cardiol. 2011;51:665–73.
Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–91.
Munson MJ, Mathai BJ, Ng MYW, Trachsel-Moncho L, de la Ballina LR, Simonsen A. GAK and PRKCD kinases regulate basal mitophagy. Autophagy. 2022;18:467–9.
Munson MJ, Mathai BJ, Ng MYW, Trachsel-Moncho L, de la Ballina LR, Schultz SW, et al. GAK and PRKCD are positive regulators of PRKN-independent mitophagy. Nat Commun. 2021;12:6101.
Zhang D, Pan J, **ang X, Liu Y, Dong G, Livingston MJ, et al. Protein Kinase Cδ Suppresses Autophagy to Induce Kidney Cell Apoptosis in Cisplatin Nephrotoxicity. J Am Soc Nephrol. 2017;28:1131–44.
Wu D, Pan J, Zhang D. Inhibition of PKC-δ reduce rhabdomyolysis-induced acute kidney injury. J Cell Mol Med. 2022;26:3243–53.
Li X, Pabla N, Wei Q, Dong G, Messing RO, Wang CY, et al. PKC-delta promotes renal tubular cell apoptosis associated with proteinuria. J Am Soc Nephrol. 2010;21:1115–24.
Galvan DL, Green NH, Danesh FR. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92:1051–7.
Yao L, Liang X, Qiao Y, Chen B, Wang P, Liu Z. Mitochondrial dysfunction in diabetic tubulopathy. Metabolism. 2022;131:155195.
Szeto HH. Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD. J Am Soc Nephrol. 2017;28:2856–65.
Doke T, Susztak K. The multifaceted role of kidney tubule mitochondrial dysfunction in kidney disease development. Trends Cell Biol. 2022;32:841–53.
Skopelja-Gardner S, An J, Elkon KB. Role of the cGAS-STING pathway in systemic and organ-specific diseases. Nat Rev Nephrol. 2022;18:558–72.
Ding C, Song Z, Shen A, Chen T, Zhang A. Small molecules targeting the innate immune cGAS‒STING‒TBK1 signaling pathway. Acta Pharm Sin B. 2020;10:2272–98.
Zheng J, Mo J, Zhu T, Zhuo W, Yi Y, Hu S, et al. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer. 2020;19:133.
Yu CH, Davidson S, Harapas CR, Hilton JB, Mlodzianoski MJ, Laohamonthonkul P, et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell. 2020;183:636–49.e18.
Aarreberg LD, Esser-Nobis K, Driscoll C, Shuvarikov A, Roby JA, Gale M Jr. Interleukin-1β Induces mtDNA Release to Activate Innate Immune Signaling via cGAS-STING. Mol Cell. 2019;74:801–15.e6.
Maekawa H, Inoue T, Ouchi H, Jao TM, Inoue R, Nishi H, et al. Mitochondrial Damage Causes Inflammation via cGAS-STING Signaling in Acute Kidney Injury. Cell Rep. 2019;29:1261–73.e6.
Li J, Sun X, Yang N, Ni J, **e H, Guo H, et al. Phosphoglycerate mutase 5 initiates inflammation in acute kidney injury by triggering mitochondrial DNA release by dephosphorylating the pro-apoptotic protein Bax. Kidney Int. 2023;103:115–33.
Zang N, Cui C, Guo X, Song J, Hu H, Yang M, et al. cGAS-STING activation contributes to podocyte injury in diabetic kidney disease. iScience. 2022;25:105145.
Yang X, Chen Z, Luo Z, Yang D, Hao Y, Hu J, et al. STING deletion alleviates podocyte injury through suppressing inflammation by targeting NLRP3 in diabetic kidney disease. Cell Signal. 2023;109:110777.
Chung KW, Dhillon P, Huang S, Sheng X, Shrestha R, Qiu C, et al. Mitochondrial Damage and Activation of the STING Pathway Lead to Renal Inflammation and Fibrosis. Cell Metab. 2019;30:784–99.e5.
Hu H, Guo L, Overholser J, Wang X. Mitochondrial VDAC1: A Potential Therapeutic Target of Inflammation-Related Diseases and Clinical Opportunities. Cells. 2022;11:3174.
**an H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55:1370–85.e8.
Kim J, Gupta R, Blanco LP, Yang S, Shteinfer-Kuzmine A, Wang K, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366:1531–6.
Jeong JJ, Park N, Kwon YJ, Ye DJ, Moon A, Chun YJ. Role of annexin A5 in cisplatin-induced toxicity in renal cells: molecular mechanism of apoptosis. J Biol Chem. 2014;289:2469–81.
Nowak G, Megyesi J, Craigen WJ. Deletion of VDAC1 Hinders Recovery of Mitochondrial and Renal Functions After Acute Kidney Injury. Biomolecules. 2020;10:585.
Li X, Pan J, Li H, Li G, Liu B, Tang X, et al. DsbA-L interacts with VDAC1 in mitochondrion-mediated tubular cell apoptosis and contributes to the progression of acute kidney disease. EBioMedicine. 2022;76:103859.
Jiang A, Liu J, Wang Y, Zhang C. cGAS-STING signaling pathway promotes hypoxia-induced renal fibrosis by regulating PFKFB3-mediated glycolysis. Free Radic Biol Med. 2023;208:516–29.
Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–96.
Wu NN, Wang L, Wang L, Xu X, Lopaschuk GD, Zhang Y, et al. Site-specific ubiquitination of VDAC1 restricts its oligomerization and mitochondrial DNA release in liver fibrosis. Exp Mol Med. 2023;55:269–80.
Shoshan-Barmatz V, Shteinfer-Kuzmine A, Verma A. VDAC1 at the Intersection of Cell Metabolism, Apoptosis, and Diseases. Biomolecules. 2020;10:1485.
Miao LN, Pan D, Shi J, Du JP, Chen PF, Gao J, et al. Role and Mechanism of PKC-δ for Cardiovascular Disease: Current Status and Perspective. Front Cardiovasc Med. 2022;9:816369.
Xu X, Pan J, Li H, Li X, Fang F, Wu D, et al. Atg7 mediates renal tubular cell apoptosis in vancomycin nephrotoxicity through activation of PKC-δ. FASEB J. 2019;33:4513–24.
Mima A, Kitada M, Geraldes P, Li Q, Matsumoto M, Mizutani K, et al. Glomerular VEGF resistance induced by PKCδ/SHP-1 activation and contribution to diabetic nephropathy. FASEB J. 2012;26:2963–74.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–53.
Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M, et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989–97.
Chichger H, Vang A, O’Connell KA, Zhang P, Mende U, Harrington EO, et al. PKC δ and βII regulate angiotensin II-mediated fibrosis through p38: a mechanism of RV fibrosis in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015;308:L827–36.
Xu X, Sun S, **e F, Ma J, Tang J, He S, et al. Advanced Oxidation Protein Products Induce Epithelial-Mesenchymal Transition of Intestinal Epithelial Cells via a PKC δ-Mediated, Redox-Dependent Signaling Pathway. Antioxid Redox Signal. 2017;27:37–56.
Jimenez SA, Gaidarova S, Saitta B, Sandorfi N, Herrich DJ, Rosenbloom JC, et al. Role of protein kinase C-delta in the regulation of collagen gene expression in scleroderma fibroblasts. J Clin Invest. 2001;108:1395–403.
Li Y, Yuan Y, Huang ZX, Chen H, Lan R, Wang Z, et al. GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy. Cell Death Differ. 2021;28:2333–50.
He Y, Deng B, Liu S, Luo S, Ning Y, Pan X, et al. Myeloid Piezo1 Deletion Protects Renal Fibrosis by Restraining Macrophage Infiltration and Activation. Hypertension. 2022;79:918–31.
Lemos DR, McMurdo M, Karaca G, Wilflingseder J, Leaf IA, Gupta N, et al. Interleukin-1β Activates a MYC-Dependent Metabolic Switch in Kidney Stromal Cells Necessary for Progressive Tubulointerstitial Fibrosis. J Am Soc Nephrol. 2018;29:1690–705.
Srivastava SP, Zhou H, Setia O, Liu B, Kanasaki K, Koya D, et al. Loss of endothelial glucocorticoid receptor accelerates diabetic nephropathy. Nat Commun. 2021;12:2368.
Taguchi S, Azushima K, Yamaji T, Urate S, Suzuki T, Abe E, et al. Effects of tumor necrosis factor-α inhibition on kidney fibrosis and inflammation in a mouse model of aristolochic acid nephropathy. Sci Rep. 2021;11:23587.
Inoue T, Takenaka T, Hayashi M, Monkawa T, Yoshino J, Shimoda K, et al. Fibroblast expression of an IκB dominant-negative transgene attenuates renal fibrosis. J Am Soc Nephrol. 2010;21:2047–52.
Wu M, Han W, Song S, Du Y, Liu C, Chen N, et al. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol Cell Endocrinol. 2018;478:115–25.
Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, et al. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–24.
Ramnath RD, Sun J, Bhatia M. PKC δ mediates pro-inflammatory responses in a mouse model of caerulein-induced acute pancreatitis. J Mol Med. 2010;88:1055–63.
Paul BD, Snyder SH, Bohr VA. Signaling by cGAS-STING in Neurodegeneration, Neuroinflammation, and Aging. Trends Neurosci. 2021;44:83–96.
Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W, et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022;52:102305.
Yan M, Li Y, Luo Q, Zeng W, Shao X, Li L, et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022;8:258.
Han W, Du C, Zhu Y, Ran L, Wang Y, **ong J, et al. Targeting Myocardial Mitochondria-STING-Polyamine Axis Prevents Cardiac Hypertrophy in Chronic Kidney Disease. JACC Basic Transl Sci. 2022;7:820–40.
Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–20.
Verma A, Pittala S, Alhozeel B, Shteinfer-Kuzmine A, Ohana E, et al. The role of the mitochondrial protein VDAC1 in inflammatory bowel disease: a potential therapeutic target. Mol Ther. 2022;30:726–44.
Shin EJ, Hwang YG, Sharma N, Tran HQ, Dang DK, Jang CG, et al. Role of protein kinase Cδ in dopaminergic neurotoxic events. Food Chem Toxicol. 2018;121:254–61.
Shin EJ, Shin SW, Nguyen TT, Park DH, Wie MB, Jang CG, et al. Ginsenoside Re rescues methamphetamine-induced oxidative damage, mitochondrial dysfunction, microglial activation, and dopaminergic degeneration by inhibiting the protein kinase Cδ gene. Mol Neurobiol. 2014;49:1400–21.
Shin EJ, Jeong JH, Nguyen BT, Sharma N, Nah SY, Chung YH, et al. Ginsenoside Re Protects against Serotonergic Behaviors Evoked by 2,5-Dimethoxy-4-iodo-amphetamine in Mice via Inhibition of PKCδ-Mediated Mitochondrial Dysfunction. Int J Mol Sci. 2021;22:7219.
** Y, Liu R, **e J, **ong H, He JC, Chen N. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab Invest. 2013;93:801–11.
Bronner DN, O’Riordan MX. Measurement of Mitochondrial DNA Release in Response to ER Stress. Bio Protoc. 2016;6:e1839.
**ao JJ, Liu Q, Li Y, Peng FF, Wang S, Zhang Z, et al. Regulator of calcineurin 1 deletion attenuates mitochondrial dysfunction and apoptosis in acute kidney injury through JNK/Mff signaling pathway. Cell Death Dis. 2022;13:774.
Acknowledgements
We thank Dr. Lin Mu (Second Hospital of Hebei Medical University, China.) for assistance with human tissues sample acquisition.
Funding
This study was supported by grants of National Natural Science Foundation of China (No. 81470966, 82300804), Guiding Local Scientific and Technological Development by the Central Government of China (No. 216Z7703G), Natural Science Foundation of Hebei Province (No. H2021206144, H2023206292), and Hebei Province Graduate Innovation Funding Project (No. CXZZBS2024116).
Author information
Authors and Affiliations
Contributions
DW: Conceptualization, Methodology, Formal analysis, Writing-original draft. YL: Methodology, Formal analysis. GL: Methodology, Validation. ML: Investigation, Resources. SS: Methodology. YB: Methodology, Data curation, Visualization. YD: Investigation, Resources, Visualization. MW: Writing-review & editing, Validation. ZZ: Methodology. JD: Methodology, Visualization. XL: Methodology, Validation. TZ: Investigation, Resources. YS: Conceptualization, Funding acquisition, Project administration, Supervision, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were reviewed and approved by Laboratory Animal Ethical and Welfare Committee of Hebei Medical University (No. IACUC-Hebmu-2021030). The research program on human tissues were approved by the Medical Ethics Committee of Hebei Medical University (No. 2021033).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Li, Y., Li, G. et al. Inhibition of PKC-δ retards kidney fibrosis via inhibiting cGAS-STING signaling pathway in mice. Cell Death Discov. 10, 314 (2024). https://doi.org/10.1038/s41420-024-02087-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-024-02087-z
- Springer Nature Limited