Abstract
Mitochondria are the centers of energy and material metabolism, and they also serve as the storage and dispatch hubs of metal ions. Damage to mitochondrial structure and function can cause abnormal levels and distribution of metal ions, leading to cell dysfunction and even death. For a long time, mitochondrial quality control pathways such as mitochondrial dynamics and mitophagy have been considered to inhibit metal-induced cell death. However, with the discovery of new metal-dependent cell death including ferroptosis and cuproptosis, increasing evidence shows that there is a complex relationship between mitochondrial quality control and metal-dependent cell death. This article reviews the latest research results and mechanisms of crosstalk between mitochondrial quality control and metal-dependent cell death in recent years, as well as their involvement in neurodegenerative diseases, tumors and other diseases, in order to provide new ideas for the research and treatment of related diseases.

Similar content being viewed by others
Facts
-
Mitochondrial quality control is a double-edged sword for cell death.
-
Metal ions not only initiate cell death by inducing oxidative stress, but also affect the regulation of cell death.
-
Mitophagy inhibits cell death via attenuation of oxidative stress, but it may also promote cell death by releasing metal ions.
-
Mitochondrial quality control has a potential effect on cuproptosis.
Questions
-
Will the ROS produced by damaged mitochondria isolated from mitophagosomes affect mitophagosomes and other organelles?
-
How different metal ions interfere with cell death induced by other metal ions?
-
How do mitophagy-caused metal ion release and mitochondrial clearance affect cuproptosis?
Introduction
Cell death is currently described on the basis of three modalities associated with different morphological characteristics: apoptosis, autophagy, and necrosis [1, 2]. Ferroptosis is a recently discovered form of regulated cell death (RCD) and is described as nonapoptotic, caspase-independent cell death mod accompanied by glutathione depletion [3,4,5,6]. It is mediated by iron overload, which results in reactive oxygen species (ROS) accumulation, glutathione depletion, lipid peroxidation, and ultimately cell death [7]. Metal ions are essential nutrients for host homeostasis and are involved in many physiological processes, while excessive or insufficient metal ions may lead to cell dysfunction and death [8]. Cuproptosis is caused by the binding of copper ions to lipoylated components of the tricarboxylic acid cycle, which in turn results in protein toxicity [9]. Both ferroptosis and cuproptosis show close connections to mitochondria: cuproptosis depends on lipoylated mitochondrial enzymes and the loss of Fe-S clusters [8]; the main characteristic of ferroptosis is lipid peroxidation, which is a result of excessive ROS production [10]. Mitochondria are metabolic centers of cellular energy, providing sufficient energy for cells through a series of metabolic mechanisms, including the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) [11]. Mitochondria plays pivotal roles in physiological functions such as fatty acid synthesis and signal processing, which further affect the fate of cells [12]. Some metal ions have been demonstrated to participate in the formation of proteins and cofactors related to mitochondrial function, and mitochondria themselves are storage centers for various metal ions in cell, which makes a close relationship between mitochondria and metal ions: the normal function of mitochondria depends on sufficient metal ion supply, and the abnormality of mitochondrial structure and function will lead to the abnormality of metal ion level and distribution [13].
Mitochondrial quality is critically involved in metal ion-dependent cell death. Multiple pathways for mitochondrial quality control regulate mitochondrial biogenesis to meet cellular metabolic, energy, and material needs through mitochondrial dynamics, mitophagy, and proteasome-mediated degradation to clear damaged or excessive mitochondria [14]. Experimental data indicated that mitophagy and ferroptosis are simultaneous processes, with mitophagy often accompanied by alterations in the expression of mitochondrial fission and fusion-related proteins such as dynamin-related protein 1 (DRP1) and mitofusin (MFN) 1/2 [15]. Moreover, the experimental results proved that mitophagy could protect cells from ferroptosis [16]. Nevertheless, increasing evidence suggests that the relationship of mitochondrial quality control with metal ion-dependent cell death is not simply inhibitory but multifactorial, involving complex interactions in the pathogenesis of various diseases [17].
Mitochondrial damage and the maintenance of mitochondrial homeostasis
Mitochondria composed of lipid bilayer membranes efficiently provide energy for utilization by eukaryotic cells [18, 19]. Metabolite intermediates or other specific macromolecules leverage porins and outer membrane transporters (TOM) to cross the mitochondrial membrane into the inner mitochondrial space (IMS) or mitochondrial matrix, regulating the TCA cycle and oxidative respiratory chain [20]. The proton motive force established by protons entering the IMS and the electron transfer mediated by OXPHOS constitute the electrochemical gradient needed for subsequent metabolic regulation, calcium buffering, and other physiological processes [21]. Mitochondrial DNA (mtDNA) and ribosomes are critically involved in synthesizing and processing peptides and proteins. Although these mtDNA-encoded products account for only a small fraction of all mitochondrial proteins, they participate in the electron transport chain (ETC) and are crucial for OXPHOS [22].
ROS: the primary mediator of mitochondrial damage
The cell nucleus regulates mitochondrial function via anterograde signaling, which regulates the expression of OXPHOS-related genes and activation of PPARγ coactivator 1α (PGC-1α) [23, 24]. Other organelles can also be subjected to retrograde regulation [25]. When mammalian cells acquire ATP synthesis abnormalities, mitochondria initiate a high-energy stress response, stimulating the AMPK/PGC-1α pathway and undergoing changed mitochondrial biogenesis [26]. Compared to the two aforementioned regulatory pathways, ROS are often considered biomarkers of mitochondrial damage, and ROS only at relatively low levels induce retrograde signaling [23]. Bidirectional communication to and from mitochondria ensures proper signal transduction, which is beneficial for maintaining calcium homeostasis and protein biogenesis [27].
Damaged mitochondria may become an unnecessary energy burden for the cell [28]. ROS are common inducers of mitochondrial damage [10]. Mitochondria can increase the inner membrane DHA/EPA ratio to enhance the electron transfer rate and NAD+/NADH ratio, thereby reducing electron leakage and ROS formation [29, 30]. Yeasts degrade ROS by expressing genes such as CTT1 and CTA1 [31, 32]. Under physiological conditions, low levels of ROS generated by mitochondria can reversibly post-translationally modify specific targets through oxidation, as a signal to regulate the body’s metabolic process. For example, when the amount of mtROS produced by mitochondria changes due to hypoxia, mtROS enhances anaerobic respiration by stabilizing HIF-1α and up-regulating key enzymes of glycolysis such as lactate dehydrogenase, reduces the dependence of cells on OXPHOS during hypoxia, and reduces the further production of mtROS. In addition, mtROS can also directly act on some proteins on the mitochondrial matrix or mitochondrial membrane, and regulate the activity of mitochondrial complex I, complex III, and complex IV through Toll-like receptors, retinoic acid-inducible gene I-like receptors and other signaling pathways, thereby affecting OXPHOS. When the ROS generation rate exceeds the clearance rate, excessive oxidation of ROS might directly damage mitochondrial lipid membranes, proteins, and mtDNA, contributing to mitochondrial dysfunction. In addition, ultraviolet light, ionizing radiation, and drug stimulation can damage mitochondria. As damaged organelles accumulate, subsequent mitochondrial failure may result in cell death [10].
Mitochondrial biogenesis and protein quality control
Mitochondrial quantity and quality regulation are achieved through mitochondrial biogenesis, mitochondrial dynamics, the degradation of misfolded proteins or damaged mitochondria through mechanism that involve including fission, fusion, and mitophagy (Fig. 1) [11, 33, 34].
Mitochondrial midzone fission is carried out under the action of DRP1 and MFF, new mitochondria are generated by biogenesis. The ER and actin synergistically determine the division site. Some mitochondria rely on MFN and OPA1 to realize the connection and fusion of bilayer membranes to meet the metabolic needs in special cases. Mitochondria are separated by peripheral fission under the action of DRP1 and lysosomes. The damaged mitochondria are transported out of the cell by mitocytosis, or degraded by lysosomes in mitophagy. The graph was created with BioRender.com. DRP1 dynamin-related protein 1, ER endoplasmic reticulum, IMM inner mitochondrial membrane, IMS intermembrane space, MFN mitofusins, OMM outer mitochondrial membrane, OPA1 optic atrophy protein 1.
Mitochondrial fission, including midzone and peripheral fission, is regulated by bacteria-derived DRPs, which assemble to hydrolyze GTP and thus affect mitochondrial membrane contents [35, 36]. Midzone fission is coordinated with mitochondrial biogenesis and contributes to the formation of new mitochondria to meet the needs of basal metabolism and cell proliferation rates. The ER determines the division site through a synergistic action with actin and then recruits mitochondrial fission factors (MFFs) and DRP1 to the cytoplasm [37]. As GTP is hydrolyzed, mitochondria are broken at the division site, forming two new daughter mitochondria, which are sites for subsequent protein assembly [21]. When alterations in nutritional status or mitochondrial dysfunction induce changes in the NAD+/NADH and AMP/ATP ratios, the expression of the PGC-1 coactivator family of proteins increases, and these proteins interact with nuclear respiratory factor (NRF)1 and 2, thereby activating the phosphorylation of AMPK and upregulating mitochondrial gene expression, transcription and replication [38, 69, 70]. With the involvement of SNARE proteins, mitophagosomes combine with lysosomes to form autolysosomes, ultimately degrading damaged mitochondria. To ensure mitophagy execution, signaling pathways in addition to the ubiquitination and PINK1-PRKN pathways, are involved. OMM proteins such as NIX, FUNDC1, and BNIP3 can act as mitophagy-related receptors, relying on their LIR domain to bind with LC3, thereby directing dysfunctional mitochondria to autophagosomes for degradation via receptor-mediated mitophagy [71].
The mechanisms underlying mitochondrial quality control do not function independently but depend on interplay with each other. For example, Ubx2 and Msp1 undergo functional interactions [48]. UPRmt can upregulate the expression of Sesn2 to increase the mitophagy rate [72]. These linked molecules form a tightly knit defense network that helps to limit mitochondrial damage. However, our current understanding of mitochondrial quality control is in nascent stages. In 2021, Yu et al. discovered a new mitochondrial quality control mode called mitocytosis, in which abnormal mitochondria are released into the extracellular space through migrasomes [73]. Contractile fibers are left behind during cell migration, and membrane-bound structures at the fiber tips or branching points form migrasomes. Damaged mitochondria are transported to the cell periphery under the influence of the KIF5B protein, entering migrasomes and eventually detaching from the cell along with the moving migrasomes [74]. These discoveries offer new perspectives for the study of mitochondrial quality control, and other currently unknown mitochondrial quality control pathways need to be discovered and characterized.
Mitochondria and iron
Mitochondria and iron metabolism
Food-derived trivalent iron is reduced to divalent iron in the duodenum and then transported into intestinal epithelial cells by the divalent metal transporter 1 (DMT1) [75, 76]. Free iron in cells is released through the basolateral membrane via ferroportin and is oxidized into trivalent iron by iron oxidases such as hephaestin, after which it is transported and utilized by transferrin (Fig. 3). Iron exists in almost all cells in the form of stable ferritin and labile iron pools (LIPs), serving as a cofactor or substrate for various proteins involved in critical biological functions, including DNA replication and lipid synthesis.
Fe3+ is transported into the cell by TfR1 and reduces to Fe2+ via Dcybt. Extracellular Fe2+ is transported into cells by DMT1 or ZIP8, then transfers to mitochondria as ferritin or with endosomes. Iron in IMS enters the mitochondrial matrix through MFRN and is processed into the Fe-S cluster or MTFT to participate in physiological activities such as energy metabolism. Extracellular copper enters cells through DMT1 or CTR1, then processes into CuL and enters mitochondria via TOM to exert related physiological functions. Zinc and calcium in the cytoplasm are transported into mitochondria through MCU, and the former and copper are involved in synthesizing key enzymes such as SOD1. The graph was created with BioRender.com. ATP adenosine triphosphate, CCS copper chaperone for superoxide dismutase, COX17 cytochrome c oxidase subunit 17/cytochrome c oxidase copper chaperone, CTR1 copper transporter 1, Dcytb duodenal cytochrome b, DMT1 divalent metal transporter 1, ETC electron transport chain, MCU Ca2+ uniporter, MFRN mitoferrins, MTFT mitochondrial ferritin, SLC25A3 solute carrier family 25 member 3, SOD1 superoxide dismutase [Cu-Zn], TfR1 transferrin receptor 1, ZIP8 zinc transporter 8.
Mitochondria are crucial in iron utilization. Iron in the cytoplasm is transported into mitochondria through endosomes and mitoferrins, crossing the OMM and IMM and subsequently serving as a substrate for synthesizing different iron-containing proteins [77,78,79]. One of the main pathways for mitochondrial iron utilization is the Fe-S cluster pathway. Fe-S cluster proteins are widely involved in multiple cellular processes, making their synthesis and transport highly conserved across cells [78]. Iron and sulfur provided via LYR motif-containing protein 4 (LYRM4) form a [2Fe-2S] cluster facilitated by iron-sulfur cluster assembly enzyme (ISCU) serving as a scaffold protein. The [2Fe-2S] cluster is released from the core ISC complex on the ISCU dimer under the action of chaperone protein HSPA9, transferred to the glutaredoxin GLRX5 dimer and synthesized by cytoplasmic iron-sulfur assembly [4Fe-4s] cluster [80, 81].
In addition to the Fe-S cluster, heme is an essential iron-containing compounds in the body, serving as a cofactor in the formation of key proteins such as hemoglobin, myoglobin, and cytochrome C, and is highly conserved, similar to the Fe-S cluster [82]. Heme is synthesized from Fe2+ and protoporphyrin IX through enzymatic reactions coordinated in the cytoplasm and mitochondria [83]. Not all iron entering mitochondria is used to synthesize Fe-S clusters or heme; excess iron is stored in mitochondria after binding with mitochondrial ferritin (MTFT) to maintain mitochondrial iron homeostasis. However, some studies have shown that MTFT overexpression affects the synthesis of Fe-S clusters and heme. Further exploration is needed to understand the regulation of mitochondrial iron content and the interaction among MTFT, Fe-S cluster, and heme [78, 84].
Iron-induced cell death
Compared to that in the LIP, free iron preferentially enters mitochondria, making mitochondria primary storage sites for irons [85]. Iron deficiency can affect the synthesis of Fe-S clusters, heme, and other proteins, thereby interfering with normal cellular functions. Transition metal elements such as iron have strong redox activity, which is closely related to the production of ROS. ROS is the main factor of endogenous oxidative stress in cells and has potential harm to mitochondria [78]. The H2O2 produced by mitochondrial inner membrane protein complex I has strong hydrophilic and lipophilic properties, allowing it to permeate the unsaturated regions of the mitochondrial membrane. In a microenvironment with a high concentration of H2O2, free iron undergoes Fenton and Haber-Weiss reactions, which generate strong oxidizing products [86]. The following equations explain these reactions:
There is ongoing debate in the academic community regarding the conditions and specific process of the Haber-Weiss reaction, i.e., reaction (2) [87, 88]. However, free iron in mitochondria and the cytoplasm can generate highly oxidative HO•, which damages polyunsaturated fatty acids (PUFAs) in the phospholipid bilayer, leading to mitochondrial outer membrane permeabilization (MOMP), ultimately abrogating DNA stability and inducing cell death. Oxidative stress caused by iron can result in various forms of cell death, including apoptosis, pyroptosis, necroptosis, and ferroptosis [89, 90].
The intrinsic pathway of apoptosis is activated by many stressors [91]. When ROS-induced MOMP occurs, cytochrome C enters the cytoplasm and is linked to apoptotic protease-activating factor-1, followed by the activation and release of caspase-3/9, which in turn contributes to morphological changes such as apoptotic body formation, chromatin condensation, and DNA fragmentation [92]. Similar to apoptosis, pyroptosis and necroptosis are induced by caspases. In contrast to apoptosis, pyroptosis and necroptosis involve the leakage of cellular contents, which can activate the proinflammatory response and are classified as lytic forms of cell death.
Pyroptosis, mediated by gasdermin D (GSDMD), differs from other RCDs due to its caspase-1 dependence and inflammatory properties [93, 94]. When pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) stimulate the activation of the NF-κB signaling pathway, inflammasomes assemble. These inflammasomes bind to pro-caspase-1 that has been released into the cytoplasm, which is then cleaved into caspase-1, activating GSDMD and driving the release of inflammatory cytokines. Inflammatory cytokines cleave the N-terminal sequence of GSDMD, and this fragment binds to the cell membrane, forming pores and inducing cell rupture, ultimately leading to pyroptosis [95, 96]. The nonclassical pathways of pyroptosis mainly depend on caspase-4/5/11; these caspases are activated by direct interaction with inflammatory stimuli such as lipopolysaccharide (LPS), which cleave and activate GSDMD, initiating pyroptosis [95, 97].
Necrosis was previously defined as type III cell death and is widely recognized as a form of accidental cell death. It is a kind of RCD similar to necrosis in terms of cell morphology, which includes cell swelling, membrane rupture, chromatin condensation, and the induction of inflammatory mediators. The classical necroptosis pathway is also the tumor necrosis receptor pathway. Death receptors (e.g., TNFR and Fas), Toll-like receptors, and cytosolic nucleic acid sensors form an autocrine feedback loop [98], recruiting proteins such as TRADD and the linear ubiquitin chain assembly complex, which further interact with caspase-8 and RIPK1 to promote RIPK1 ubiquitination. After stimulation by the relevant signals, RIPK1 undergoes ubiquitination, recruits RIPK3, forms the RIPK1/RIPK3 complex, mediates MLKL oligomerization and forms specific necrosomes in the cytoplasm, thereby leading to pore formation on the plasma membrane [89] with cell swelling and membrane rupture. Activated RIPK3 can induce mtROS production by binding to the E3 subunit of the pyruvate dehydrogenase complex [99]. During infection, GSDMD binds to the mitochondrial membrane to form pores, releasing mtROS and promoting RIPK1/RIPK3/MLKL-dependent necroptosis [100].
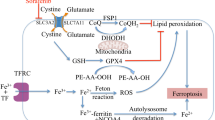
In contrast to the aforementioned RCD types, ferroptosis exhibits higher dependence on transition metals, particularly iron, and it does not require caspase action but relies on the oxidative activity of Fe2+, which is its main distinguishing feature. The morphological changes in ferroptotic cells are largely concentrated in the mitochondria, including mitochondrial shrinkage, increased mitochondrial membrane density and rupture, and reduced mitochondrial cristae. Ferroptosis is essentially the outcome of oxidative stress caused by iron overload, with Fe2+ and PUFAs and lipid peroxidation the leading cause of ferroptotic cell death [101, 102]. PUFAs are added to phospholipids (PLs) via the esterification action of long-chain fatty acid CoA ligase 4 (ACSL4), which enters the membrane to generate PUFA-PLs. PUFAs react with the products of the Fenton reaction, producing phospholipid hydroperoxides (PLOOH) after dehydrogenation. Fe2+ is not only the leading participant in the Fenton reaction during ferroptosis but also causes ferroptosis through other programs. arachidonate lipoxygenases (ALOXs) catalyze the oxidation of PUFAs to generate hydroperoxy PUFA derivatives. Because ALOXs are a nonheme iron-containing enzymes, the presence of iron significantly increases their oxidative activity, and the subsequent generation of PLOOH continues to react with Fe2+, generating new lipid radicals. When the central repressors of ferroptosis, such as glutathione (GSH) and lipid enzyme glutathione peroxidase 4 (GPX4) [103], which participate in peroxide reduction and reduce product toxicity, show insufficient activity, the lipid radicals formed cannot be cleared and continue to generate new oxidative products via chain reaction. The accumulated peroxidized lipids eventually destroy the membrane structure and cause cell death [104].
Most of the iron-induced cell death is caused by ROS produced by Fenton reaction [90, 105, 106]. Interestingly, there are cases of iron-dependent death unrelated to ROS in fungi; notably, some fungi exhibit growth inhibition under high levels of cytoplasmic iron, and this phenotype is not associated with antioxidant enzymes [107]. Although these outcomes have been found only in fungi, they suggest unique relationships between metal ions and cell death and indicate that oxidation is not the only factor causing cell damage. Accumulating evidence has shown that some proteins involved in cell death, especially death receptors, are directly regulated by iron ions: the key death receptor Fas in apoptosis is expressed in two isoforms, an anti-apoptotic and a pro-apoptotic form, and iron is the key regulator in Fas exon splicing. An increase in iron content switches Fas from being an anti-apoptotic protein to being a pro-apoptotic protein, thereby activating the extrinsic apoptosis pathway and promoting necroptosis [91]. Nevertheless, the list of direct impacts of iron on cell death that have been discovered to date is still incomplete, and further exploration is required.
Mitochondrial quality control and iron-induced cell death: a double-edged sword
Mitochondria are highly susceptible to the impacts of the iron-mediated Fenton reaction. Mechanisms underlying mitochondrial quality control include inhibition of ROS production within mitochondria, a decrease in the accumulation of abnormal proteins, and prevention of iron-induced cell death. For example, in the case of mitochondrial fusion, the overexpression of Fzo1A/B or MFN1/2 prevents excessive fragmentation of mitochondria. These fragmented mitochondria increase cellular sensitivity to apoptotic stimuli, while enhanced mitochondrial fusion significantly reduces the occurrence of MOMP, thereby inhibiting cell apoptosis [108]. Similarly, in cells under oxidative stress conditions, activated NRF2 can enhance the expression of MFN1 and MFN2 while degrading DRP2 through the proteasomal pathway, leading to mitochondrial hyperfusion, which temporarily protects cells and alleviates oxidative stress as well as inhibits ferroptosis [109]. Mitochondria undergoing imbalanced fusion and fission cannot maintain normal function for an extended period; when the accumulated damage exceeds the range tolerated for mitochondrial fusion, mitochondria are fragmented, triggering mitophagy to prevent further damage [110].
Even targeting the GSH/GPX4 antioxidant pathways may initiate several types of cell death, including ferroptosis. Many members of the HSP family can counteract oxidation through the FSP1/CoQ10 axis and other pathways; for example, HSP70 upregulates GPX4 expression to prevent ferroptosis induced by lipid oxidation [111]. HSP90 directly interacts with GPX4, inhibiting GPX4 activity and resulting in ferroptosis [112]. Additionally, HSP90 regulates signaling pathways such as the RIPK1 and RIPK3 signaling pathways; HSP90 inhibitors can significantly suppress necroptotic cell death [113].
Mitochondria are among the primary sources of ROS [114]. Compared to those of the mitochondrial quality control system, the characteristics of mitophagy, which completely degrades mitochondrial quality control system substrates, make it the ultimate program to manage damaged mitochondria, playing inhibitory and remediating roles in iron-induced cell death, such as ferroptosis (Fig. 4). Experimental evidence showed that activating mitophagy through genetic or pharmacological approaches, especially in the early stages of oxidative stress, can significantly reduce the risk of ferroptosis by clearing and degrading damaged mitochondria, possibly by preventing subsequent metabolic abnormalities from generating excess ROS [211,212].
Vascular diseases and related diseases
The bidirectional effect caused by mitochondrial quality control is not unique to infectious diseases such as sepsis. Vascular diseases involve multiple tissues and organs, and thrombosis is the basis of various vascular diseases. Mitochondrial quality control and iron metabolism are important in thrombosis. High levels of ROS can directly stimulate platelet activation and aggregation or can activate platelets through the cell adhesion factor P-selectin [213]. Interestingly, each of these processes can be inhibited by iron chelators [214]. Additionally, GPXs downregulate platelet-dependent thrombosis, and GSH consumption induced by lipid peroxidation attenuates this response [215]. Although mitophagy limits iron release to a certain extent, the impact of the iron that is released remains unknown. A study showed that improving platelet energy and material supply function by mitophagy promoted platelet activation (Table 1) [216]. Kawasaki disease (KD) is a common self-limiting form of pediatric vasculitis that often involves coronary arteries due to thrombosis. Vascular smooth muscle cells (VSMCs) in children with Kawasaki disease can increase ROS levels due to abnormal autophagy processes, activating corresponding cell death pathways and upregulating expression of NLRP3, to exacerbate mitochondrial dysfunction and vascular inflammation [217]. Studies revealed that serum iron levels are often reduced and serum ferritin levels are abnormally high in patients with KD, and these effects can be mediated by hepcidin treatment [218, 219]. Through the combined action of various factors, VSMCs undergo iron overload and death, affecting vascular endothelial integrity. In addition, thymic stromal lymphopoietin is significantly elevated in patients, and it induces platelet activation via mitophagy agonists, contributing to thrombosis [220].
Atherosclerosis is another common disease caused by abnormal VSMCs and platelets; it is closely related to mitochondrial quality control in various cells and is often accompanied by apoptosis, pyroptosis, and ferroptosis of macrophages as well as endothelial cells [221]. Genetic or environmental factors lead to the accumulation of cholesterol-enriched lipoproteins in the arterial wall. Mitochondrial dysfunction caused by defects in long-term quality control mechanisms can dysregulate the normal function and damage the structure of VSMCs, stimulate oxidative modification of lipoproteins in the vascular wall, activate the immune response to recruit monocytes, and induce cells to differentiate into macrophages that engulf retained lipoproteins [222, 223]. Macrophages or smooth muscle cells that accumulate too much lipid are transformed into foam cells, which are more susceptible to iron overload [224]. Inhibition of autophagy in cells under high-fat conditions aggravates atherosclerosis, and iron accumulation often occurs in plaques, which may be caused by dysregulated mitophagy and the abrogation of the fragile metabolic balance in foam cells [225, 226]. Notably, mitochondrial quality control does not play a protective role in atherosclerosis. Experimental results demonstrated that apelin-13, an endogenous ligand of the G protein-coupled receptor angiotensin II protein J (APJ), increased DRP1 expression, inhibited fusion-related protein expression, and stimulated VSMC mitophagy and abnormal proliferation, which exacerbated the development of atherosclerosis [227]. Cells on the vascular wall continued to proliferate, aggregate, die, and accumulate to form atheromatous plaques. When the plaque ruptured, platelets form thrombi block the lumen, which is naturally narrow, subsequently causing ischemia and hypoxia in affected organs.
Hypoxia promotes the conversion of cells from aerobic respiration to anaerobic respiration. In addition to stimulating calcium overload caused by ATP enzyme imbalances, such as imbalances in calcium pump activity, metabolites, including lactic acid and succinic acid, accumulate, antioxidant enzyme activity is inhibited, the mitochondrial structure is destroyed and mitochondrial function is dysregulated under ischemic conditions. After blood flow recovery, mitochondrial permeability increases because of the high-level calcium, and moreover, succinate, mtDNA, and other substances are released. As DAMPs, these secreted factors activate the apoptotic pathway and autophagy and induce oxidative stress, and the antioxidant mechanism cannot be restored before apoptosis and ferroptosis are induces [228]. The heart is vulnerable to damage caused by metal overload and mitochondrial dysfunction due to the metabolic characteristics of cardiomyocytes [225, 229]. Most studies suggest that mitophagy exhibits a protective effect on the myocardium. Overexpression of Drp1 and Atg5 in cardiomyocytes or inhibition of p53 to increase autophagic flux can prevent symptoms such as myocardial hypertrophy and aging [230, 231]. In contrast, hypoxia stimulates FUNDC1-mediated mitophagy, but this process does not play a protective role after paraquat exposure, and it aggravates ferroptosis and apoptosis in cardiomyocytes [232]. After treatment with the mitochondrial inhibitor mdivi-1 or knocking down BNIP3, the myocardial infarct size related to mitophagy was significantly diminished [233, 234]. A similar response was found in the case of renal injury following an ischemia-reperfusion episode. Ischemia-reperfusion injury-activated mitophagy establishes a positive feedback loop involving apoptosis with proteins such as MEG3, which aggravates acute kidney injury that is secondary to ischemia-reperfusion injury [235]. Obviously, similar to the results of infectious diseases, mitochondrial quality control, e.g., activation of mitophagy, can indeed confer protection to blood vessels, myocardium, and kidney cells, but the damage caused by mitochondrial quality control is unpredictable [236]. Ways to make rational use of this response to control disease activity within a limited range, a remarkable challenge, need to be identified.
Metabolic diseases
Changes in modern lifestyles and eating habits have led to a sharp increase in patients with metabolic diseases [237]. Obesity and diabetes are the most common types of metabolic diseases. These diseases can lead to a variety of related diseases, including cardiovascular disease and cancer, which impose great burdens to health system worldwide. Obesity is one of the main risk factors for metabolic diseases such as diabetes and non-alcoholic fatty liver disease (NAFLD) and is often accompanied by abnormal mitochondrial function and mitochondrial quality control. Compared with that in healthy individual, the expression of citrate synthase, which is the rate-limiting enzyme in the TCA cycle, is reduced in obese patients, which inhibits glucose and lipid metabolism and the normal energy supply of mitochondria and increases ROS production [238]. The expression levels of the mitochondrial fusion proteins MFN2 and OPA1 and the mitophagy-related protein p62 in obese patients is also significantly decreased, resulting in an imbalance in mitochondrial fusion, fission and clearance, which seriously affects mitochondrial quality [239,240,241]. In addition, a high-fat diet can significantly increase the level of caspase-3 and decrease the level of bcl-2, which increases the susceptibility of cells to apoptosis and causes a series of obesity-related complications [242, 243].
In addition to obesity, diabetes is another common metabolic disorder, and damage to islet β cells is the main cause of diabetes. The physiological characteristics of β cells and the high glucose environment associated with diabetes make these cells highly susceptible to death induced by iron and other metal ions. Previous studies have shown that in diabetic patients, the pancreas, especially islet β cells, is often characterized by excessive iron accumulation, and the level of antioxidant enzymes in β cells is low, which makes these cells susceptible to oxidative stress [244, 245]. Iron overload decreases glucose oxidation and increases fatty acid oxidation, which gradually leads to insulin resistance. Increased blood glucose levels induce excessive mitochondrial fission or fragmentation, thereby affecting OXPHOS, inhibit the expression of PINK1 and Parkin and downregulate the expression of genes such as PGC-1α, thereby inhibiting mitochondrial biogenesis and mitophagy and preventing cells from eliminating abnormal mitochondria. This increases ROS production and lipid accumulation through peroxidation, resulting in apoptosis and ferroptosis [246,247,248]. Recent studies have shown that the CLEC16A gene plays a protective role in type 1 diabetes by regulating β-cell mitophagy. The Clec16a protein encodes an E3 ubiquitin ligase, and the complex of this factor with Nrdp1 and Usp8 can promote the fusion of mitophagosomes and lysosomes. In an inflammatory state, knockout of Clec16a in β cells can significantly increase apoptosis and sharply increase blood glucose levels in patients, and hyperglycemia further affects mitochondrial quality control, promotes cell damage, and exacerbates diabetes. The accumulation of human amylin in pancreatic islets is a typical feature of type 2 diabetes. Amyloid overexpression stimulates mTORC1 signaling, inhibits mitophagy, and increases apoptosis. Amyloid protein aggregates can also form cytotoxic oligomers, destroy the integrity of the cell membrane, and further aggravate islet damage [249, 250]. A high-glucose environment affects islet β cells and mitochondrial quality control in the nervous system, cardiovascular system, urinary system cells and gradually causes common complications such as diabetic peripheral neuropathy, diabetic cardiomyopathy and diabetic nephropathy.
NAFLD is a common complication of obesity and diabetes. Excessive triglyceride and glucose levels can place a burden on the liver and cause liver fat infiltration. The accumulation of fat in the liver gradually progresses from initial steatosis to steatohepatitis and eventually progresses to cirrhosis [251]. Mitochondrial dysfunction and metal metabolism disorders are important causes of NAFLD [252]. Hyperferritinemia and liver iron deposition occur in NAFLD patients, which may be related to the increase in intestinal iron uptake and the decrease in liver cell iron efflux in NAFLD patients [253, 254]. During the early stage of NAFLD, liver cells exhibit increases iron levels, oxidative stress and ferroptosis-related phospholipids and decreased mitopahgy [255,256,257]. The use of ferroptosis inhibitors can reduce hepatocyte death and inflammation during NAFLD and alleviate the progression of NAFLD. In addition, the key ferroptosis factor frataxin not only plays a role in neurological diseases but is also involved in the occurrence and development of NAFLD. Early studies confirmed that frataxin deficiency could lead to obesity in mice. In the response to a high-fat diet and free fatty acids, the level of frataxin in the liver is significantly reduced. Activation of frataxin can enhance PINK1/Parkin-mediated mitophagy, which can significantly ameliorate lipid accumulation induced by a high-fat diet and free fatty acids [258, 259].
Iron is not the only metal ion involved in metabolic diseases. Abnormal Ca2+ levels caused by mitochondrial Ca2+ uptake disorders have also been shown to be involved in the pathogenesis of type 2 diabetes [260]. In addition, higher serum copper levels are associated with obesity and diabetes, and increased copper levels have been observed in the liver cells of NAFLD patients, which suggests that copper can induce these metabolic diseases. However, in some interventional studies, copper has been shown to exert a protective effect on diabetic patients, and a restricted copper diet can also induce steatosis in the liver [261,262,263]. The specific role of copper and other metals, their ability to induce cell death in metabolic diseases and the mechanism need to be further studied.
Musculoskeletal diseases
Bones and muscles maintain bodily functions such as breathing, eating and movement. Damage to these tissues can seriously affect patient quality of life and may cause more serious diseases and endanger life. Osteoporosis (OP) is a common motor system disease caused by an imbalance between bone resorption and bone formation. Mitochondria are the key factors that maintain the balance of activity between osteoblasts and osteoclasts. Abnormal mitochondrial quality control can lead to changes in the activity of osteoblasts and osteoclasts. An increase is ROS caused by various factors stimulates the expression of DRP1 in osteoblasts. Abnormal mitochondrial fission leads to mitochondrial dysfunction or fragmentation, which affects the function of osteoblasts. Moreover, abnormal mitochondria further aggravate the production of ROS, resulting in positive feedback. However, in osteoclasts, DRP1 overexpression promotes osteoclast differentiation [264]. In addition, when the proportions of S-OPA1 and L-OPA1, which mediate mitochondrial fusion are out of balance, mitochondrial fusion in osteoblasts is inhibited, and abnormal mitochondria produce large amounts of mtROS, thereby inducing osteoblast apoptosis [265]. Abnormal iron metabolism in mitochondria can also aggravate the progression of OP. A lack of MTFT induces mitophagy in osteoblasts through the ROS/PINK1/Parkin pathway to release iron, but excess the iron cannot be stored through the MTFT pathway, gradually accumulates and ultimately induces ferroptosis in osteoblasts [266]. However, some studies have shown that the absence of PINK1 leads to a significant reduction in bone mass in patients. How to regulate mitophagy to an appropriate level to maintain the balance between osteoclasts and osteoblasts is still a problem [267].
Osteoarthritis (OA) is a chronic disabling disease caused by articular cartilage and bone injury. Experimental results revealed obvious oxidative stress in chondrocytes during the development of OA, which was related to abnormal iron metabolism, mitochondrial metabolic disorders and other factors [268]. The proinflammatory cytokine IL-1β can increase TfR1 and inhibit FPN expression, promote iron transport into the cell and reduce iron efflux, and excessive iron causes mitochondrial dysfunction through the Fenton reaction. Furthermore, the expression of DRP1 was upregulated, resulting in the fragmentation of mitochondria and exacerbated oxidative stress [269]. In chondrocytes from mice with IL-1β-induced osteoarthritis, decreased protein expression of GPX4 and SLC7A11 and lipid ROS accumulation were observed, which inhibited the expression of type II collagen in chondrocytes and affected bone and joint health. Ferrostatin-1, which is a ferroptosis inhibitor, can alleviate these symptoms [270]. In chondrocytes from humans with osteoarthritis, IL-1β not only inhibited chondrocyte proliferation but also induced apoptosis. After treatment with α-ketoglutarate (α-KG), mitophagy flux increased, mitochondrial structure and function significantly improved, ROS production was inhibited, and OA was improved through the alleviation of chondrocyte apoptosis [268].
Satellite cells (SCs), which are located under the basal layer of muscle fiber, are critical for skeletal muscle regeneration, maintaining a reversible static state and activating myoblasts when needed. With age, SCs undergo irreversible senescence, which seriously affects the repair function of SCs and leads to sarcopenia [271]. A decrease in the number and function of SCs is associated with mitochondrial damage and metal-induced cell death. In aging SCs, mitochondrial fission, mitophagy efficiency, and the OXPHOS energy supply are low [272]. In a sarcopenia mouse model, muscle iron levels and ferroptosis markers were significantly increased, and GPX4 was downregulated. The use of the ferroptosis inhibitors Ferr-1 and DFO could significantly ameliorate these symptoms. In addition, age-related iron accumulation was observed in a model constructed with C2C12 myoblasts [273]. Experiments have shown that adiponectin, which is secreted by adipocytes, can significantly reduce the expression of PINK1 and Parkin induced by oxidative stress, thereby inhibiting mitophagy and apoptosis caused by mitophagy in C2C12 myoblasts, which provides a new idea for the diagnosis and treatment of skeletal muscle diseases such as sarcopenia and early-onset myopathies [274].
Prospects and challenges
In recent years, with the discovery of cell death modes such as ferroptosis and copper death, we have gained a deeper understanding of metal ion-induced cell death. However, investigations are still in their infancy, and many scientific issues need to be resolved. Different metal ions exert shared influence on their transport and utilization. Various metal nutrients are obtained via intake of food, and most of them are transported from the intestine to intestinal epithelial cells or from the cells to the intestinal lumen, and both processes require DMT1. Inhibition of DMT1 expression reduces the transport of iron, copper, and zinc into cells, suggesting that DMT1 mediates the transport of multiple metals [275]. However, the number of transporters is limited and they can be saturated, and there is competitive inhibition between the transport processes of multiple metal ions [276]. When too much metal is ingested, the transport of other metals is affected. For example, silver plays a competitive role in mitochondrial copper absorption, significantly inhibiting the uptake of copper by mitochondria and influencing the subsequent COX assembly process [277]. In contrast, ceruloplasmin is involved in iron transport. Iron transport can be disrupted when copper levels are profoundly low or ceruloplasmin levels are too low, leading to gradual iron accumulation in tissues [160]. The specific impacts of the interaction between different metals on cells remain unclear, and various metal ions may be involved in the same cell death process. It has been reported that iron plays a role in copper-mediated ferroptosis, and the use of copper ion chelating agents does not abate iron accumulation in ferroptosis [278]. These findings raise new questions: what is the relationship between different cell death pathways in the presence of multiple metals, and how is the signaling that leads to the final death of the cell mediated? Unfortunately, in metal ion-mediated cell death, we have focus only on concentration changes of a certain metal ion and gave ignored the possible effects of other metal ions.
In addition, cell death induced by the overload of the same metal seems to be nonspecific; notably, death caused by metal-mediated oxidative stress is a common cause of a variety of types of cell death. Previous research results may provide possible explanations. First, identical proteins may exert opposite effects on different cell death pathways. For example, FSP1 is an NAD(P)H-dependent oxidoreductase that has long been regarded as a proapoptotic factor that synergistically triggers apoptosis with 4-hydroxy-2-none under oxidative stress conditions [279]. However, recent studies have shown that FSP1 inhibited ferroptosis by capturing lipid peroxide radicals on the membrane via coenzyme Q10 [280]. Second, the metal itself seems to have the ability to inhibit certain key enzymes in death pathways; for example, copper can cause the downregulation of other forms of cell death except cuproptosis, as mentioned above [146, 159]. Third, various types of cell death, such as ferroptosis, can be rapidly induced in a cascade among adjacent cells, which may be mediated by cell-cell contact. Gaps and tight junctions diffuse specific cytokines from dead cells to other cells and induce the corresponding cell death in a specific range of cells [281, 282]. Nevertheless, evidence is rare, and identical proteins may play roles in promoting different cell death processes. ELAV-like protein 1 (ELAVL1), encoding the RNA-binding protein HuR, has been shown to induce pyroptosis in cardiomyocytes under hyperglycemic conditions, and recent data revealed that upregulated ELAVL1 induced classical ferroptosis events [283, 284].
How the specific pathway is activated needs to be clarified to deepen the understanding of the mechanism underlying various death modalities. Similarly, the function of mitochondria in cell death deserves in-depth study. In addition to ROS formation and metal ion release, attention should be paid to the potential role of mitochondria themselves. Due to the involvement of the TCA cycle, the relationship between mitochondria and the cuproptosis pathway appears to be unquestionable, but researchers have different opinions on the role of mitochondria in ferroptosis. It is believed that ferroptosis is induced by lipid peroxidation outside mitochondria, and there is a situation in which mitochondria and mtDNA in cells are depleted but ferroptosis still occurs [6, 285, 286]. Although mitochondria do not play important roles in RSL3-induced ferroptosis, the evidence suggests that the mitochondrial ETC is indispensable for erastin-induced or cystine deprivation-mediated ferroptosis. Moreover, GPX4 in mitochondria exerts a more significant effect on cell resistance to ferroptosis than that exerted by GPX4 in the cytoplasm, and mitochondrial events seem to be final steps in the determination of whether cells undergo ferroptosis [287, 288]. What potential role do mitochondria play in the pathogenesis of ferroptosis? The answer to this question is of great importance to elucidate the mechanisms underlying mitochondrial quality control in ferroptosis. There may be two different pathways, a mitochondrial-dependent and mitochondrial-independent pathways. Through various pathways, “maintenance” based on mitochondrial dynamics and protein quality control and “clearance” represented by mitocytosis and mitophagy may lead to different outcomes.
Mitochondria are not isolated organelles; when the protein synthesis and transport to organelles are hindered, the function of mitochondria is affected. Imbalances in the homeostasis of metal ions, such as calcium, can influence mitochondrial function and induce cell death via ER stress. Various mechanisms underlying mitochondrial protein quality control depend on the participation of ERAD and UPS to maintain mitochondrial function as the first line of defense, and mitochondrial fusion and fission rely on the ER and lysosomes. The pathway of ferritinophagy, another autophagy modality directly affected by intracellular iron ion concentration, engages in crosstalk with the mitophagy pathway. Inhibiting the glucose flux sensing modification O-GlcNAcylation increased the rates of mitophagy and ferritinophagy, both of which released a large amount of free iron and jointly induced ferroptosis [116]. Notably, in ferroptosis, an increase in ferritinophagy activation and a decrease in mitophagy activation were noted, despite the continuous elevation in intracellular iron content, mitochondrial quantity, and ROS [289]. In addition, lipophagy, in which lipid droplets are eliminated, and clockophagy, which is based on the circadian rhythm protein ARNTL, play potential roles in ferroptosis and are associated to some degree with mitophagy [290, 291]. Although research into autophagy has been ongoing for a considerable period, we still know little about it, and the specific connections among different types of autophagy are unclear. Different types of selective autophagy depend to a certain extent on identical proteins and organelles, and lysosomes are required for the final step, which is substrate degradation. Do different autophagy pathways compete for shared resources to simultaneously interfere with each other?
The regulatory effect of metal ions on mitochondrial quality control may also be involved in cell death. Metal-induced oxidative stress caused by redox imbalances is a direct factor in the activation of mitochondrial quality control. ROS produced by the Fenton reaction promote mitochondrial hyperfusion or activate the mitochondrial fission-autophagy degradation pathway. In addition, iron and other metal ions are cofactors of many proteins. Many key proteins that regulate mitochondrial function and mitochondrial quality control, including the mitochondrial OXPHOS complex, require the participation of iron, copper and other metal elements. The Fe-S cluster is widely involved in a variety of physiological processes, and normal biogenesis of this factor is essential for life. Iron deficiency-induce Fe-S synthesis disorders affect the voltage-dependent anion channel in the mitochondrial membrane, interferes with the overall stability of mitochondria, and induces apoptosis [292]. Copper ions are involved in autophagy and are essential for the activity of autophagy kinases such as MEK1/2 and ULK1/2. Recent studies have shown that MIRO1, which induces mitochondrial membrane protrusions in MDVs, is regulated by Fe2+ and Ca2+, which provides new ideas for research in related fields [293]. Unfortunately, there is a gap in the study of the interactions between mitochondrial quality control and metal ions, the effect of metal ions on mitochondrial function has rarely been studied, and most of the relevant studies have focus only on mitochondrial dynamics and oxidative stress. There is a long way to go to apply related theories to clinical trials and human therapy.
Conclusions
Abnormal metal ion metabolism can lead to various types of cell death. Mitochondria are centers of energy metabolism and material transport and play key roles in regulating metal ion-induced cell death. Maintaining homeostasis through mitochondrial quality control is essential for antagonizing metal ion-induced cell death. However, altering mitochondrial quality control may also promote cell death, making mitochondrial quality control a double-edged sword. In various diseases, there is a contradictory relationship between mitochondrial quality control and metal-induced cell death, and these processes have a delicate balance. For example, in neurodegenerative diseases, infections and other diseases, physiological levels of mitophagy can protect cells from pathogenic factors, but long-term uncontrolled mitochondrial quality control can exacerbate disease-related damage. In tumors, the balance between mitochondrial quality control and metal-induced cell death must be disrupted to promote tumor cell death. In fact, there are many potential factors that can modulate this balance, and it has broad application prospects in clinical practice. Future research can be expanded in related fields to provide more results and verify our hypotheses, thereby opening new paths to treat diseases.
Data availability
The datasets used and analyzed during the current study are available from corresponding author on reasonable request.
References
Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–43.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–20.
Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharm Exp Ther. 1973;187:211–7.
Sordillo LM, Weaver JA, Cao YZ, Corl C, Sylte MJ, Mullarky IK. Enhanced 15-HPETE production during oxidant stress induces apoptosis of endothelial cells. Prostaglandins Other Lipid Mediat. 2005;76:19–34.
Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–48.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.
Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022;32:417–8.
Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–61.
Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843.
Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5.
Cunningham CN, Rutter J. 20,000 picometers under the OMM: diving into the vastness of mitochondrial metabolite transport. EMBO Rep. 2020;21:e50071.
Medlock AE, Hixon JC, Bhuiyan T, Cobine PA. Prime real estate: metals, cofactors and MICOS. Front Cell Dev Biol. 2022;10:892325.
Youle RJ. Mitochondria-Striking a balance between host and endosymbiont. Science. 2019;365:eaaw9855.
Wen S, Hu X, Shi Y, Han J, Han S. Imaging of mitophagy enabled by an acidity-reporting probe anchored on the mitochondrial inner membrane. Anal Chem. 2021;93:16887–98.
Pei Z, Liu Y, Liu S, ** W, Luo Y, Sun M, et al. FUNDC1 insufficiency sensitizes high fat diet intake-induced cardiac remodeling and contractile anomaly through ACSL4-mediated ferroptosis. Metabolism. 2021;122:154840.
Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol. 2020;27:420–35.
Endo T, Sakaue H. Multifaceted roles of porin in mitochondrial protein and lipid transport. Biochem Soc Trans. 2019;47:1269–77.
Rapaport D. Biogenesis of the mitochondrial TOM complex. Trends Biochem Sci. 2002;27:191–7.
Herrmann JM, Riemer J. The intermembrane space of mitochondria. Antioxid Redox Signal. 2010;13:1341–58.
Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–43.
Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–23.
Quirós PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol. 2016;17:213–26.
Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–66.
Arnould T, Vankoningsloo S, Renard P, Houbion A, Ninane N, Demazy C, et al. CREB activation induced by mitochondrial dysfunction is a new signaling pathway that impairs cell proliferation. EMBO J. 2002;21:53–63.
Garcia-Roves PM, Osler ME, Holmström MH, Zierath JR. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J Biol Chem. 2008;283:35724–34.
Banerjee K, Keasey MP, Razskazovskiy V, Visavadiya NP, Jia C, Hagg T. Reduced FAK-STAT3 signaling contributes to ER stress-induced mitochondrial dysfunction and death in endothelial cells. Cell Signal. 2017;36:154–62.
Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20:233–42.
Aviello G, Knaus UG. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol. 2018;11:1011–23.
Hagopian K, Weber KL, Hwee DT, Van Eenennaam AL, López-Lluch G, Villalba JM, et al. Complex I-associated hydrogen peroxide production is decreased and electron transport chain enzyme activities are altered in n-3 enriched fat-1 mice. PLoS One. 2010;5:e12696.
Hartig A, Ruis H. Nucleotide sequence of the Saccharomyces cerevisiae CTT1 gene and deduced amino-acid sequence of yeast catalase T. Eur J Biochem. 1986;160:487–90.
Cohen G, Fessl F, Traczyk A, Rytka J, Ruis H. Isolation of the catalase A gene of Saccharomyces cerevisiae by complementation of the cta1 mutation. Mol Gen Genet. 1985;200:74–79.
Moos WH, Faller DV, Glavas IP, Harpp DN, Kamperi N, Kanara I, et al. Treatment and prevention of pathological mitochondrial dysfunction in retinal degeneration and in photoreceptor injury. Biochem Pharm. 2022;203:115168.
Pickles S, Vigié P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28:R170–R185.
Faelber K, Gao S, Held M, Posor Y, Haucke V, Noé F, et al. Oligomerization of dynamin superfamily proteins in health and disease. Prog Mol Biol Transl Sci. 2013;117:411–43.
Kleele T, Rey T, Winter J, Zaganelli S, Mahecic D, Perreten Lambert H, et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature. 2021;593:435–9.
Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–62.
Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–22.
Qian X, Li X, Shi Z, Bai X, **a Y, Zheng Y, et al. KDM3A senses oxygen availability to regulate PGC-1α-mediated mitochondrial biogenesis. Mol Cell. 2019;76:885–95.
Bouchez C, Devin A. Mitochondrial biogenesis and mitochondrial reactive oxygen species (ROS): a complex relationship regulated by the cAMP/PKA signaling pathway. Cells. 2019;8:287.
Herrmann JM, Longen S, Weckbecker D, Depuydt M. Biogenesis of mitochondrial proteins. Adv Exp Med Biol. 2012;748:41–64.
Qin J, Guo Y, Xue B, Shi P, Chen Y, Su QP, et al. ER-mitochondria contacts promote mtDNA nucleoids active transportation via mitochondrial dynamic tubulation. Nat Commun. 2020;11:4471.
Boos F, Krämer L, Groh C, Jung F, Haberkant P, Stein F, et al. Mitochondrial protein-induced stress triggers a global adaptive transcriptional programme. Nat Cell Biol. 2019;21:442–51.
Hwang J, Qi L. Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends Biochem Sci. 2018;43:593–605.
Ng MYW, Wai T, Simonsen A. Quality control of the mitochondrion. Dev Cell. 2021;56:881–905.
Karbowski M, Youle RJ. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol. 2011;23:476–82.
Weidberg H, Amon A. MitoCPR-A surveillance pathway that protects mitochondria in response to protein import stress. Science. 2018;360:eaan4146.
Mårtensson CU, Priesnitz C, Song J, Ellenrieder L, Doan KN, Boos F, et al. Mitochondrial protein translocation-associated degradation. Nature. 2019;569:679–83.
Hirsch C, Gauss R, Horn SC, Neuber O, Sommer T. The ubiquitylation machinery of the endoplasmic reticulum. Nature. 2009;458:453–60.
Wu X, Li L, Jiang H. Doa1 targets ubiquitinated substrates for mitochondria-associated degradation. J Cell Biol. 2016;213:49–63.
Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–9.
Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell. 2007;13:467–80.
McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33:282–95.
Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol. 2012;22:135–41.
Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, et al. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol. 2008;18:102–8.
König T, Nolte H, Aaltonen MJ, Tatsuta T, Krols M, Stroh T, et al. MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control. Nat Cell Biol. 2021;23:1271–86.
Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–80.
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200.
Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–62.
Li YJ, Cao YL, Feng JX, Qi Y, Meng S, Yang JF, et al. Structural insights of human mitofusin-2 into mitochondrial fusion and CMT2A onset. Nat Commun. 2019;10:4914.
Gao S, Hu J. Mitochondrial fusion: the machineries in and out. Trends Cell Biol. 2021;31:62–74.
Zhang D, Zhang Y, Ma J, Zhu C, Niu T, Chen W, et al. Cryo-EM structures of S-OPA1 reveal its interactions with membrane and changes upon nucleotide binding. Elife. 2020;9:e50294.
Yan L, Qi Y, Ricketson D, Li L, Subramanian K, Zhao J, et al. Structural analysis of a trimeric assembly of the mitochondrial dynamin-like GTPase Mgm1. Proc Natl Acad Sci USA. 2020;117:4061–70.
Wong YC, Ysselstein D, Krainc D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature. 2018;554:382–6.
Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27:105–17.
Pryde KR, Smith HL, Chau KY, Schapira AH. PINK1 disables the anti-fission machinery to segregate damaged mitochondria for mitophagy. J Cell Biol. 2016;213:163–71.
Kondapalli C, Kazlauskaite A, Zhang N, Woodroof HI, Campbell DG, Gourlay R, et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012;2:120080.
Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19:4861–70.
Lamark T, Svenning S, Johansen T. Regulation of selective autophagy: the p62/SQSTM1 paradigm. Essays Biochem. 2017;61:609–24.
Kirkin V, Rogov VV. A Diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol Cell. 2019;76:268–85.
Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40:e104705.
Wang Y, Jasper H, Toan S, Muid D, Chang X, Zhou H. Mitophagy coordinates the mitochondrial unfolded protein response to attenuate inflammation-mediated myocardial injury. Redox Biol. 2021;45:102049.
Jiao H, Jiang D, Hu X, Du W, Ji L, Yang Y, et al. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell. 2021;184:2896–910.
Mehra C, Pernas L. Move it to lose it: mitocytosis expels damaged mitochondria. Dev Cell. 2021;56:2014–5.
Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8.
Ma C, Han L, Zhu Z, Heng Pang C, Pan G. Mineral metabolism and ferroptosis in non-alcoholic fatty liver diseases. Biochem Pharm. 2022;205:115242.
Hamdi A, Roshan TM, Kahawita TM, Mason AB, Sheftel AD, Ponka P. Erythroid cell mitochondria receive endosomal iron by a “kiss-and-run” mechanism. Biochim Biophys Acta. 2016;1863:2859–67.
Ward DM, Cloonan SM. Mitochondrial iron in human health and disease. Annu Rev Physiol. 2019;81:453–82.
Liu Q, Wu J, Zhang X, Wu X, Zhao Y, Ren J. Iron homeostasis and disorders revisited in the sepsis. Free Radic Biol Med. 2021;165:1–13.
Lill R. From the discovery to molecular understanding of cellular iron-sulfur protein biogenesis. Biol Chem. 2020;401:855–76.
Rouault TA, Maio N. Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J Biol Chem. 2017;292:12744–53.
Ponka P, Sheftel AD, English AM, Scott Bohle D, Garcia-Santos D. Do mammalian cells really need to export and import heme? Trends Biochem Sci. 2017;42:395–406.
Dailey HA, Meissner PN. Erythroid heme biosynthesis and its disorders. Cold Spring Harb Perspect Med. 2013;3:a011676.
Nie G, Sheftel AD, Kim SF, Ponka P. Overexpression of mitochondrial ferritin causes cytosolic iron depletion and changes cellular iron homeostasis. Blood. 2005;105:2161–7.
Crielaard BJ, Lammers T, Rivella S. Targeting iron metabolism in drug discovery and delivery. Nat Rev Drug Discov. 2017;16:400–23.
Qi X, Zhang Y, Guo H, Hai Y, Luo Y, Yue T. Mechanism and intervention measures of iron side effects on the intestine. Crit Rev Food Sci Nutr. 2020;60:2113–25.
Filipovic MR, Koppenol WH. The Haber-Weiss reaction: the latest revival. Free Radic Biol Med. 2019;145:221–2.
Koppenol WH. A resurrection of the Haber-Weiss reaction. Nat Commun. 2022;13:396.
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64.
Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17.
Nakamura T, Naguro I, Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta Gen Subj. 2019;1863:1398–409.
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52.
Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–4.
Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–44.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–21.
Yang Z, Wang Y, Zhang Y, He X, Zhong CQ, Ni H, et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat Cell Biol. 2018;20:186–97.
Weindel CG, Martinez EL, Zhao X, Mabry CJ, Bell SL, Vail KJ, et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell. 2022;185:3214–31.
Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89–100.
Tang D, Kroemer G. Ferroptosis. Curr Biol. 2020;30:R1292–R1297.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31.
Zhang S, Sun Z, Jiang X, Lu Z, Ding L, Li C, et al. Ferroptosis increases obesity: crosstalk between adipocytes and the neuroimmune system. Front Immunol. 2022;13:1049936.
Regdon Z, Demény MA, Kovács K, Hajnády Z, Nagy-Pénzes M, Bakondi E, et al. High-content screening identifies inhibitors of oxidative stress-induced parthanatos: cytoprotective and anti-inflammatory effects of ciclopirox. Br J Pharm. 2021;178:1095–113.
Robinson N, Ganesan R, Hegedűs C, Kovács K, Kufer TA, Virág L. Programmed necrotic cell death of macrophages: focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019;26:101239.
Li L, Chen OS, McVey Ward D, Kaplan J. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J Biol Chem. 2001;276:29515–9.
Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–34.
Chen QM. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic Biol Med. 2022;179:133–43.
Tian C, Liu Y, Li Z, Zhu P, Zhao M. Mitochondria related cell death modalities and disease. Front Cell Dev Biol. 2022;10:832356.
Liu Y, Zhou L, Xu Y, Li K, Zhao Y, Qiao H, et al. Heat shock proteins and ferroptosis. Front Cell Dev Biol. 2022;10:864635.
Chen C, Wang D, Yu Y, Zhao T, Min N, Wu Y, et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021;12:65.
Li D, Xu T, Cao Y, Wang H, Li L, Chen S, et al. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci USA. 2015;112:5017–22.
Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–63.
Li Y, Wang X, Huang Z, Zhou Y, **a J, Hu W, et al. CISD3 inhibition drives cystine-deprivation induced ferroptosis. Cell Death Dis. 2021;12:839.
Yu F, Zhang Q, Liu H, Liu J, Yang S, Luo X, et al. Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov. 2022;8:40.
Li C, Liu J, Hou W, Kang R, Tang D. STING1 promotes ferroptosis through MFN1/2-dependent mitochondrial fusion. Front Cell Dev Biol. 2021;9:698679.
Rademaker G, Boumahd Y, Peiffer R, Anania S, Wissocq T, Liégeois M, et al. Myoferlin targeting triggers mitophagy and primes ferroptosis in pancreatic cancer cells. Redox Biol. 2022;53:102324.
Basit F, van Oppen LM, Schöckel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716.
Allen GF, Toth R, James J, Ganley IG. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 2013;14:1127–35.
Rakshit J, Priyam A, Gowrishetty KK, Mishra S, Bandyopadhyay J. Iron chelator deferoxamine protects human neuroblastoma cell line SH-SY5Y from 6-Hydroxydopamine-induced apoptosis and autophagy dysfunction. J Trace Elem Med Biol. 2020;57:126406.
Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL, Cheng K, et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28:1171–85.
Scott TL, Wicker CA, Suganya R, Dhar B, Pittman T, Horbinski C, et al. Polyubiquitination of apurinic/apyrimidinic endonuclease 1 by Parkin. Mol Carcinog. 2017;56:325–36.
Long K, Gu L, Li L, Zhang Z, Li E, Zhang Y, et al. Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer. Cell Death Dis. 2021;12:503.
McNeill DR, Narayana A, Wong HK, Wilson DM. Inhibition of Ape1 nuclease activity by lead, iron, and cadmium. Environ Health Perspect. 2004;112:799–804.
van Vuren AJ, van Beers EJ, van Wijk R. A proposed concept for defective mitophagy leading to late stage ineffective erythropoiesis in pyruvate kinase deficiency. Front Physiol. 2021;11:609103.
Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–5.
Zhang K, Wang Y, Chen S, Mao J, ** Y, Ye H, et al. TREM2hi resident macrophages protect the septic heart by maintaining cardiomyocyte homeostasis. Nat Metab. 2023;5:129–46.
Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, Spears JW, et al. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem. 2010;285:32385–92.
Chen X, Cai Q, Liang R, Zhang D, Liu X, Zhang M, et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 2023;14:105.
Festa RA, Thiele DJ. Copper: an essential metal in biology. Curr Biol. 2011;21:R877–R883.
Robinson NJ, Winge DR. Copper metallochaperones. Annu Rev Biochem. 2010;79:537–62.
Garza NM, Swaminathan AB, Maremanda KP, Zulkifli M, Gohil VM. Mitochondrial copper in human genetic disorders. Trends Endocrinol Metab. 2023;34:21–33.
Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378.
Ruiz LM, Libedinsky A, Elorza AA. Role of copper on mitochondrial function and metabolism. Front Mol Biosci. 2021;8:711227.
Petris MJ, Voskoboinik I, Cater M, Smith K, Kim BE, Llanos RM, et al. Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J Biol Chem. 2002;277:46736–42.
Ma B, Wang S, Liu F, Zhang S, Duan J, Li Z, et al. Self-assembled copper-amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J Am Chem Soc. 2019;141:849–57.
Li T, Wang D, Meng M, Yang Z, Luo Z, Li Z, et al. Copper-coordinated covalent organic framework produced a robust Fenton-like effect inducing immunogenic cell death of tumors. Macromol Rapid Commun. 2023;44:e2200929.
Kang Z, Qiao N, Liu G, Chen H, Tang Z, Li Y. Copper-induced apoptosis and autophagy through oxidative stress-mediated mitochondrial dysfunction in male germ cells. Toxicol Vitr. 2019;61:104639.
Rakshit A, Khatua K, Shanbhag V, Comba P, Datta A. Cu2+ selective chelators relieve copper-induced oxidative stress in vivo. Chem Sci. 2018;9:7916–30.
Zhou Q, Zhang Y, Lu L, Zhang H, Zhao C, Pu Y, et al. Copper induces microglia-mediated neuroinflammation through ROS/NF-κB pathway and mitophagy disorder. Food Chem Toxicol. 2022;168:113369.
Chen J, Zhang R, Tao C, Huang X, Chen Z, Li X, et al. CuS-NiS2 nanomaterials for MRI guided phototherapy of gastric carcinoma via triggering mitochondria-mediated apoptosis and MLKL/CAPG-mediated necroptosis. Nanotoxicology. 2020;14:774–87.
Gao W, Huang Z, Duan J, Nice EC, Lin J, Huang C. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol Oncol. 2021;15:3527–44.
Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023;19:1982–96.
Tsvetkov P, Detappe A, Cai K, Keys HR, Brune Z, Ying W, et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat Chem Biol. 2019;15:681–9.
Kahlson MA, Dixon SJ. Copper-induced cell death. Science. 2022;375:1231–2.
Chen X, Zhang X, Chen J, Yang Q, Yang L, Xu D, et al. Hinokitiol copper complex inhibits proteasomal deubiquitination and induces paraptosis-like cell death in human cancer cells. Eur J Pharm. 2017;815:147–55.
Fontana F, Raimondi M, Marzagalli M, Di Domizio A, Limonta P. The emerging role of paraptosis in tumor cell biology: perspectives for cancer prevention and therapy with natural compounds. Biochim Biophys Acta Rev Cancer. 2020;1873:188338.
Luzio A, Parra S, Costa B, Santos D, Álvaro AR, Monteiro SM. Copper impair autophagy on zebrafish (Danio rerio) gill epithelium. Environ Toxicol Pharm. 2021;86:103674.
Tsang T, Posimo JM, Gudiel AA, Cicchini M, Feldser DM, Brady DC. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat Cell Biol. 2020;22:412–24.
Liu H, Deng H, Cui H, Jian Z, Guo H, Fang J, et al. Copper induces hepatocyte autophagy via the mammalian targets of the rapamycin signaling pathway in mice. Ecotoxicol Environ Saf. 2021;208:111656.
Luo Q, Song Y, Kang J, Wu Y, Wu F, Li Y, et al. mtROS-mediated Akt/AMPK/mTOR pathway was involved in copper-induced autophagy and it attenuates copper-induced apoptosis in RAW264.7 mouse monocytes. Redox Biol. 2021;41:101912.
Huo Y, Ma F, Li T, Lei C, Liao J, Han Q, et al. Exposure to copper activates mitophagy and endoplasmic reticulum stress-mediated apoptosis in chicken (Gallus gallus) cerebrum. Environ Toxicol. 2023;38:392–402.
Pantoom S, Pomorski A, Huth K, Hund C, Petters J, Krężel A, et al. Direct interaction of ATP7B and LC3B proteins suggests a cooperative role of copper transportation and autophagy. Cells. 2021;10:3118.
Masaldan S, Clatworthy SAS, Gamell C, Smith ZM, Francis PS, Denoyer D, et al. Copper accumulation in senescent cells: interplay between copper transporters and impaired autophagy. Redox Biol. 2018;16:322–331.
Zhang J, Wang B, Wang H, He H, Wu Q, Qin X, et al. Disruption of the superoxide anions-mitophagy regulation axis mediates copper oxide nanoparticles-induced vascular endothelial cell death. Free Radic Biol Med. 2018;129:268–78.
Pan M, Zheng Q, Yu Y, Ai H, **e Y, Zeng X, et al. Seesaw conformations of Npl4 in the human p97 complex and the inhibitory mechanism of a disulfiram derivative. Nat Commun. 2021;12:121.
Zhang Y, Maurizi MR. Mitochondrial ClpP activity is required for cisplatin resistance in human cells. Biochim Biophys Acta. 2016;1862:252–64.
Tardito S, Bassanetti I, Bignardi C, Elviri L, Tegoni M, Mucchino C, et al. Copper binding agents acting as copper ionophores lead to caspase inhibition and paraptotic cell death in human cancer cells. J Am Chem Soc. 2011;133:6235–42.
Zeng L, Ai CX, Zhang JS, Li WC. Pre-hypoxia exposure inhibited copper toxicity by improving energy metabolism, antioxidant defence and mitophagy in the liver of the large yellow croaker Larimichthys crocea. Sci Total Environ. 2020;708:134961.
Xu Q, Zhan G, Zhang Z, Yong T, Yang X, Gan L. Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics. 2021;11:1937–52.
Wang Y, Wu T, Tang M. Ambient particulate matter triggers dysfunction of subcellular structures and endothelial cell apoptosis through disruption of redox equilibrium and calcium homeostasis. J Hazard Mater. 2020;394:122439.
Pei X, Liu D, Li J, Li L, Ding X, Zhang W, et al. TFEB coordinates autophagy and pyroptosis as hepatotoxicity responses to ZnO nanoparticles. Sci Total Environ. 2023;865:161242.
Seth R, Corniola RS, Gower-Winter SD, Morgan TJ Jr, Bishop B, Levenson CW. Zinc deficiency induces apoptosis via mitochondrial p53- and caspase-dependent pathways in human neuronal precursor cells. J Trace Elem Med Biol. 2015;30:59–65.
Baltaci AK, Yuce K, Mogulkoc R. Zinc metabolism and metallothioneins. Biol Trace Elem Res. 2018;183:22–31.
Zhang S, Kang L, Dai X, Chen J, Chen Z, Wang M, et al. Manganese induces tumor cell ferroptosis through type-I IFN dependent inhibition of mitochondrial dihydroorotate dehydrogenase. Free Radic Biol Med. 2022;193:202–12.
Kudla J, Becker D, Grill E, Hedrich R, Hippler M, Kummer U, et al. Advances and current challenges in calcium signaling. N Phytol. 2018;218:414–31.
Bauer TM, Murphy E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res. 2020;126:280–93.
Song Z, Song H, Liu D, Yan B, Wang D, Zhang Y, et al. Overexpression of MFN2 alleviates sorafenib-induced cardiomyocyte necroptosis via the MAM-CaMKIIδ pathway in vitro and in vivo. Theranostics. 2022;12:1267–85.
Du W, Gu M, Hu M, Pinchi P, Chen W, Ryan M, et al. Lysosomal Zn2+ release triggers rapid, mitochondria-mediated, non-apoptotic cell death in metastatic melanoma. Cell Rep. 2021;37:109848.
Dineley KE, Votyakova TV, Reynolds IJ. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J Neurochem. 2003;85:563–70.
Hu H, Xu Q, Mo Z, Hu X, He Q, Zhang Z, et al. New anti-cancer explorations based on metal ions. J Nanobiotechnol. 2022;20:457.
Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, et al. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2018;17:660–88.
Gan L, Cookson MR, Petrucelli L, La Spada AR. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci. 2018;21:1300–9.
Huang ML, Lane DJ, Richardson DR. Mitochondrial mayhem: the mitochondrion as a modulator of iron metabolism and its role in disease. Antioxid Redox Signal. 2011;15:3003–19.
Artyukhova MA, Tyurina YY, Chu CT, Zharikova TM, Bayır H, Kagan VE, et al. Interrogating Parkinson’s disease associated redox targets: potential application of CRISPR editing. Free Radic Biol Med. 2019;144:279–92.
Wang ZL, Yuan L, Li W, Li JY. Ferroptosis in Parkinson’s disease: glia-neuron crosstalk. Trends Mol Med. 2022;28:258–69.
Kawahara M, Kato-Negishi M, Tanaka K. Cross talk between neurometals and amyloidogenic proteins at the synapse and the pathogenesis of neurodegenerative diseases. Metallomics. 2017;9:619–33.
Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, et al. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology. 1997;48:650–8.
Davies KM, Bohic S, Carmona A, Ortega R, Cottam V, Hare DJ, et al. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging. 2014;35:858–66.
Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 2017;552:187–93.
Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol. 2012;181:1426–35.
Cecarini V, Bonfili L, Cuccioloni M, Keller JN, Bruce-Keller AJ, Eleuteri AM. Effects of ghrelin on the proteolytic pathways of Alzheimer’s disease neuronal cells. Mol Neurobiol. 2016;53:3168–78.
Sensi SL, Granzotto A, Siotto M, Squitti R. Copper and zinc dysregulation in Alzheimer’s disease. Trends Pharm Sci. 2018;39:1049–63.
Polishchuk EV, Polishchuk RS. The emerging role of lysosomes in copper homeostasis. Metallomics. 2016;8:853–62.
Cossée M, Puccio H, Gansmuller A, Koutnikova H, Dierich A, LeMeur M, et al. Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum Mol Genet. 2000;9:1219–26.
Schiavi A, Maglioni S, Palikaras K, Shaik A, Strappazzon F, Brinkmann V, et al. Iron-starvation-induced mitophagy mediates lifespan extension upon mitochondrial stress in C. elegans. Curr Biol. 2015;25:1810–22.
García-Giménez JL, Gimeno A, Gonzalez-Cabo P, Dasí F, Bolinches-Amorós A, Mollá B, et al. Differential expression of PGC-1α and metabolic sensors suggest age-dependent induction of mitochondrial biogenesis in Friedreich ataxia fibroblasts. PLoS One. 2011;6:e20666.
Capitanio D, Vasso M, De Palma S, Fania C, Torretta E, Cammarata FP, et al. Specific protein changes contribute to the differential muscle mass loss during ageing. Proteomics. 2016;16:645–56.
Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5:421–32.
Brewer GJ. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors. 2012;38:107–13.
Motawi TK, Al-Kady RH, Senousy MA, Abdelraouf SM. Repaglinide elicits a neuroprotective effect in rotenone-induced Parkinson’s disease in rats: emphasis on targeting the DREAM-ER stress BiP/ATF6/CHOP trajectory and activation of mitophagy. ACS Chem Neurosci. 2023;14:180–94.
Jiang Y, Huo Z, Qi X, Zuo T, Wu Z. Copper-induced tumor cell death mechanisms and antitumor theragnostic applications of copper complexes. Nanomedicines. 2022;17:303–24.
Denoyer D, Masaldan S, La Fontaine S, Cater MA. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics. 2015;7:1459–76.
Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. 2022;22:102–13.
**ong C, Ling H, Hao Q, Zhou X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023;30:876–84.
Yang W, Wang Y, Huang Y, Yu J, Wang T, Li C, et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to promote cuproptosis in colorectal cancer. Biomed Pharmacother. 2023;159:114301.
Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912–9.
Miao Z, Tian W, Ye Y, Gu W, Bao Z, Xu L, et al. Hsp90 induces Acsl4-dependent glioma ferroptosis via dephosphorylating Ser637 at Drp1. Cell Death Dis. 2022;13:548.
Feng Y, Xu J, Shi M, Liu R, Zhao L, Chen X, et al. COX7A1 enhances the sensitivity of human NSCLC cells to cystine deprivation-induced ferroptosis via regulating mitochondrial metabolism. Cell Death Dis. 2022;13:988.
Arezes J, Jung G, Gabayan V, Valore E, Ruchala P, Gulig PA, et al. Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe. 2015;17:47–57.
Huang ZL, Failla ML. Copper deficiency suppresses effector activities of differentiated U937 cells. J Nutr. 2000;130:1536–42.
Ueda N, Takasawa K. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients. 2018;10:1173.
Nemeth E, Ganz T. Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int J Mol Sci. 2021;22:6493.
Xu T, Dong Q, Luo Y, Liu Y, Gao L, Pan Y, et al. Porphyromonas gingivalis infection promotes mitochondrial dysfunction through Drp1-dependent mitochondrial fission in endothelial cells. Int J Oral Sci. 2021;13:28.
Mazumder S, Bindu S, De R, Debsharma S, Pramanik S, Bandyopadhyay U. Emerging role of mitochondrial DAMPs, aberrant mitochondrial dynamics and anomalous mitophagy in gut mucosal pathogenesis. Life Sci. 2022;305:120753.
Zhang Y, Chen L, Luo Y, Wang K, Liu X, **ao Z, et al. Pink1/Parkin-mediated mitophagy regulated the apoptosis of dendritic cells in sepsis. Inflammation. 2022;45:1374–87.
Li JY, Ren C, Wang LX, Yao RQ, Dong N, Wu Y, et al. Sestrin2 protects dendrite cells against ferroptosis induced by sepsis. Cell Death Dis. 2021;12:834.
Deng S, Zhang L, Mo Y, Huang Y, Li W, Peng Q, et al. Mdivi-1 attenuates lipopolysaccharide-induced acute lung injury by inhibiting MAPKs, oxidative stress and apoptosis. Pulm Pharm Ther. 2020;62:101918.
Carcillo JA, Kernan KK, Horvat CM, Simon DW, Aneja RK. Why and how is hyperferritinemic sepsis different from sepsis without hyperferritinemia? Pediatr Crit Care Med. 2020;21:509–12.
Moreira AC, Mesquita G, Gomes MS. Ferritin: an inflammatory player kee** iron at the core of pathogen-host interactions. Microorganisms. 2020;8:589.
Pei F, Yao RQ, Ren C, Bahrami S, Billiar TR, Chaudry IH, et al. Expert consensus on the monitoring and treatment of sepsis-induced immunosuppression. Mil Med Res. 2022;9:74.
Rodvein R, Lindon JN, Levine PH. Physiology and ultrastructure of the blood platelet following exposure to hydrogen peroxide. Br J Haematol. 1976;33:19–26.
NaveenKumar SK, SharathBabu BN, Hemshekhar M, Kemparaju K, Girish KS, Mugesh G. The role of reactive oxygen species and ferroptosis in heme-mediated activation of human platelets. ACS Chem Biol. 2018;13:1996–2002.
Brigelius-Flohé R, Flohé L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid Redox Signal. 2020;33:498–516.
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, et al. Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARγ/FUNDC1/mitophagy pathways. J Pineal Res. 2017;63:10.1111.
Marek-Iannucci S, Ozdemir AB, Moreira D, Gomez AC, Lane M, Porritt RA, et al. Autophagy-mitophagy induction attenuates cardiovascular inflammation in a murine model of Kawasaki disease vasculitis. JCI Insight. 2021;6:151981.
Wen H, Hun M, Zhao M, Han P, He Q. Serum ferritin as a crucial biomarker in the diagnosis and prognosis of intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease: A systematic review and meta-analysis. Front Med. 2022;9:941739.
Huang YH, Kuo HC, Huang FC, Yu HR, Hsieh KS, Yang YL, et al. Hepcidin-induced iron deficiency is related to transient anemia and hypoferremia in Kawasaki disease patients. Int J Mol Sci. 2016;17:715.
Fu L, MacKeigan DT, Gong Q, Che D, Xu Y, Pi L, et al. Thymic stromal lymphopoietin induces platelet mitophagy and promotes thrombosis in Kawasaki disease. Br J Haematol. 2023;200:776–91.
Huynh DTN, Heo KS. Role of mitochondrial dynamics and mitophagy of vascular smooth muscle cell proliferation and migration in progression of atherosclerosis. Arch Pharm Res. 2021;44:1051–61.
Sergin I, Bhattacharya S, Emanuel R, Esen E, Stokes CJ, Evans TD, et al. Inclusion bodies enriched for p62 and polyubiquitinated proteins in macrophages protect against atherosclerosis. Sci Signal. 2016;9:ra2.
Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–21.
Su G, Yang W, Wang S, Geng C, Guan X. SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1Β and IL-18. Biochem Biophys Res Commun. 2021;561:33–39.
Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120:1812–24.
Sullivan JL. Macrophage iron, hepcidin, and atherosclerotic plaque stability. Exp Biol Med. 2007;232:1014–20.
He L, Zhou Q, Huang Z, Xu J, Zhou H, Lv D, et al. PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKα and exacerbates atherosclerotic lesions. J Cell Physiol. 2019;234:8668–82.
Granata S, Votrico V, Spadaccino F, Catalano V, Netti GS, Ranieri E, et al. Oxidative stress and ischemia/reperfusion injury in kidney transplantation: focus on ferroptosis, mitophagy and new antioxidants. Antioxidants. 2022;11:769.
Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. 2011;124:2253–63.
Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–6.
Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, et al. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–87.
Peng H, Fu S, Wang S, Xu H, Dhanasekaran M, Chen H, et al. Ablation of FUNDC1-dependent mitophagy renders myocardium resistant to paraquat-induced ferroptosis and contractile dysfunction. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166448.
Gharanei M, Hussain A, Janneh O, Maddock H. Attenuation of doxorubicin-induced cardiotoxicity by mdivi-1: a mitochondrial division/mitophagy inhibitor. PLoS One. 2013;8:e77713.
Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–33.
Liu D, Liu Y, Zheng X, Liu N. c-MYC-induced long noncoding RNA MEG3 aggravates kidney ischemia-reperfusion injury through activating mitophagy by upregulation of RTKN to trigger the Wnt/β-catenin pathway. Cell Death Dis. 2021;12:191.
Su L, Zhang J, Gomez H, Kellum JA, Peng Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy. 2023;19:401–14.
Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–85.
Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13:2051–60.
Pereira RO, Tadinada SM, Zasadny FM, Oliveira KJ, Pires KMP, Olvera A, et al. OPA1 deficiency promotes secretion of FGF21 from muscle that prevents obesity and insulin resistance. EMBO J. 2017;36:2126–45.
Liu R, ** P, Yu L, Wang Y, Han L, Shi T, et al. Impaired mitochondrial dynamics and bioenergetics in diabetic skeletal muscle. PLoS One. 2014;9:e101265.
Greene NP, Lee DE, Brown JL, Rosa ME, Brown LA, Perry RA, et al. Mitochondrial quality control, promoted by PGC-1α, is dysregulated by Western diet-induced obesity and partially restored by moderate physical activity in mice. Physiol Rep. 2015;3:e12470.
Abrigo J, Rivera JC, Aravena J, Cabrera D, Simon F, Ezquer F, et al. High fat diet-induced skeletal muscle wasting is decreased by mesenchymal stem cells administration: implications on oxidative stress, ubiquitin proteasome pathway activation, and myonuclear apoptosis. Oxid Med Cell Longev. 2016;2016:9047821.
Dungan CM, Li J, Williamson DL. Caloric restriction normalizes obesity-induced alterations on regulators of skeletal muscle growth signaling. Lipids. 2016;51:905–12.
Wang J, Wang H. Oxidative stress in pancreatic beta cell regeneration. Oxid Med Cell Longev. 2017;2017:1930261.
Coffey R, Knutson MD. The plasma membrane metal-ion transporter ZIP14 contributes to nontransferrin-bound iron uptake by human β-cells. Am J Physiol Cell Physiol. 2017;312:C169–C175.
Shan Z, Fa WH, Tian CR, Yuan CS, Jie N. Mitophagy and mitochondrial dynamics in type 2 diabetes mellitus treatment. Aging. 2022;14:2902–19.
Scheele C, Nielsen AR, Walden TB, Sewell DA, Fischer CP, Brogan RJ, et al. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? FASEB J. 2007;21:3653–65.
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73.
Sidarala V, Pearson GL, Parekh VS, Thompson B, Christen L, Gingerich MA, et al. Mitophagy protects β cells from inflammatory damage in diabetes. JCI Insight. 2020;5:e141138.
Hernández MG, Aguilar AG, Burillo J, Oca RG, Manca MA, Novials A, et al. Pancreatic β cells overexpressing hIAPP impaired mitophagy and unbalanced mitochondrial dynamics. Cell Death Dis. 2018;9:481.
Raza S, Rajak S, Upadhyay A, Tewari A, Anthony Sinha R. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci (Landmark Ed). 2021;26:206–37.
Sheldon RD, Meers GM, Morris EM, Linden MA, Cunningham RP, Ibdah JA, et al. eNOS deletion impairs mitochondrial quality control and exacerbates Western diet-induced NASH. Am J Physiol Endocrinol Metab. 2019;317:E605–E616.
Rametta R, Fracanzani AL, Fargion S, Dongiovanni P. Dysmetabolic hyperferritinemia and dysmetabolic iron overload syndrome (DIOS): two related conditions or different entities? Curr Pharm Des. 2020;26:1025–35.
Hoki T, Miyanishi K, Tanaka S, Takada K, Kawano Y, Sakurada A, et al. Increased duodenal iron absorption through up-regulation of divalent metal transporter 1 from enhancement of iron regulatory protein 1 activity in patients with nonalcoholic steatohepatitis. Hepatology. 2015;62:751–61.
Tsuchiya H, Ebata Y, Sakabe T, Hama S, Kogure K, Shiota G. High-fat, high-fructose diet induces hepatic iron overload via a hepcidin-independent mechanism prior to the onset of liver steatosis and insulin resistance in mice. Metabolism. 2013;62:62–69.
Tsurusaki S, Tsuchiya Y, Koumura T, Nakasone M, Sakamoto T, Matsuoka M, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10:449.
Undamatla R, Fagunloye OG, Chen J, Edmunds LR, Murali A, Mills A, et al. Reduced mitophagy is an early feature of NAFLD and liver-specific PARKIN knockout hastens the onset of steatosis, inflammation and fibrosis. Sci Rep. 2023;13:7575.
Pomplun D, Voigt A, Schulz TJ, Thierbach R, Pfeiffer AF, Ristow M. Reduced expression of mitochondrial frataxin in mice exacerbates diet-induced obesity. Proc Natl Acad Sci USA. 2007;104:6377–81.
Liu P, Lin H, Xu Y, Zhou F, Wang J, Liu J, et al. Frataxin-mediated PINK1-Parkin-dependent mitophagy in hepatic steatosis: the protective effects of quercetin. Mol Nutr Food Res. 2018;62:e1800164.
Wang CH, Tsai TF, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca2+ homeostasis in insulin insensitivity of mammalian cells. Ann NY Acad Sci. 2015;1350:66–76.
Yang H, Liu CN, Wolf RM, Ralle M, Dev S, Pierson H, et al. Obesity is associated with copper elevation in serum and tissues. Metallomics. 2019;11:1363–71.
Eljazzar S, Abu-Hijleh H, Alkhatib D, Sokary S, Ismail S, Al-Jayyousi GF, et al. The role of copper intake in the development and management of type 2 diabetes: a systematic review. Nutrients. 2023;15:1655.
Song M, Schuschke DA, Zhou Z, Chen T, Pierce WM Jr, Wang R, et al. High fructose feeding induces copper deficiency in Sprague-Dawley rats: a novel mechanism for obesity related fatty liver. J Hepatol. 2012;56:433–40.
Zhang L, Gan X, He Y, Zhu Z, Zhu J, Yu H. Drp1-dependent mitochondrial fission mediates osteogenic dysfunction in inflammation through elevated production of reactive oxygen species. PLoS One. 2017;12:e0175262.
Cai WJ, Chen Y, Shi LX, Cheng HR, Banda I, Ji YH, et al. AKT-GSK3β signaling pathway regulates mitochondrial dysfunction-associated OPA1 cleavage contributing to osteoblast apoptosis: preventative effects of hydroxytyrosol. Oxid Med Cell Longev. 2019;2019:4101738.
Wang X, Ma H, Sun J, Zheng T, Zhao P, Li H, et al. Mitochondrial ferritin deficiency promotes osteoblastic ferroptosis via mitophagy in type 2 diabetic osteoporosis. Biol Trace Elem Res. 2022;200:298–307.
Zhao W, Zhang W, Ma H, Yang M. NIPA2 regulates osteoblast function by modulating mitophagy in type 2 diabetes osteoporosis. Sci Rep. 2020;10:3078.
Liu L, Zhang W, Liu T, Tan Y, Chen C, Zhao J, et al. The physiological metabolite α-ketoglutarate ameliorates osteoarthritis by regulating mitophagy and oxidative stress. Redox Biol. 2023;62:102663.
**g X, Du T, Li T, Yang X, Wang G, Liu X, et al. The detrimental effect of iron on OA chondrocytes: Importance of pro-inflammatory cytokines induced iron influx and oxidative stress. J Cell Mol Med. 2021;25:5671–80.
Yao X, Sun K, Yu S, Luo J, Guo J, Lin J, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Transl. 2020;27:33–43.
Wang Y, Zhang Z, Jiao W, Wang Y, Wang X, Zhao Y, et al. Ferroptosis and its role in skeletal muscle diseases. Front Mol Biosci. 2022;9:1051866.
Hong X, Isern J, Campanario S, Perdiguero E, Ramírez-Pardo I, Segalés J, et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell. 2022;29:1506–8.
Huang Y, Wu B, Shen D, Chen J, Yu Z, Chen C. Ferroptosis in a sarcopenia model of senescence accelerated mouse prone 8 (SAMP8). Int J Biol Sci. 2021;17:151–62.
Ren Y, Li Y, Yan J, Ma M, Zhou D, Xue Z, et al. Adiponectin modulates oxidative stress-induced mitophagy and protects C2C12 myoblasts against apoptosis. Sci Rep. 2017;7:3209.
Arredondo M, Muñoz P, Mura CV, Nùñez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol. 2003;284:C1525–C1530.
Arredondo M, Martínez R, Núñez MT, Ruz M, Olivares M. Inhibition of iron and copper uptake by iron, copper and zinc. Biol Res. 2006;39:95–102.
Vest KE, Leary SC, Winge DR, Cobine PA. Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J Biol Chem. 2013;288:23884–92.
Sarraf SA, Shah HV, Kanfer G, Pickrell AM, Holtzclaw LA, Ward ME, et al. Loss of TAX1BP1-directed autophagy results in protein aggregate accumulation in the brain. Mol Cell. 2020;80:779–95.
Miriyala S, Thippakorn C, Chaiswing L, Xu Y, Noel T, Tovmasyan A, et al. Novel role of 4-hydroxy-2-nonenal in AIFm2-mediated mitochondrial stress signaling. Free Radic Biol Med. 2016;91:68–80.
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8.
Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22:1042–8.
Belavgeni A, Meyer C, Stumpf J, Hugo C, Linkermann A. Ferroptosis and necroptosis in the kidney. Cell Chem Biol. 2020;27:448–62.
Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, et al. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun. 2016;471:423–9.
Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A, et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083–103.
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91.
Gaschler MM, Hu F, Feng H, Linkermann A, Min W, Stockwell BR. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem Biol. 2018;13:1013–20.
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90.
Oh SJ, Ikeda M, Ide T, Hur KY, Lee MS. Mitochondrial event as an ultimate step in ferroptosis. Cell Death Discov. 2022;8:414.
Song XY, Liu PC, Liu WW, Zhou J, Hayashi T, Mizuno K, et al. Silibinin inhibits ethanol- or acetaldehyde-induced ferroptosis in liver cell lines. Toxicol Vitr. 2022;82:105388.
**e Y, Li J, Kang R, Tang D. Interplay between lipid metabolism and autophagy. Front Cell Dev Biol. 2020;8:431.
Rabinovich-Nikitin I, Rasouli M, Reitz CJ, Posen I, Margulets V, Dhingra R, et al. Mitochondrial autophagy and cell survival is regulated by the circadian clock gene in cardiac myocytes during ischemic stress. Autophagy. 2021;17:3794–812.
Ferecatu I, Canal F, Fabbri L, Mazure NM, Bouton C, Golinelli-Cohen MP. Dysfunction in the mitochondrial Fe-S assembly machinery leads to formation of the chemoresistant truncated VDAC1 isoform without HIF-1α activation. PLoS One. 2018;13:e0194782.
Bharat V, Durairaj AS, Vanhauwaert R, Li L, Muir CM, Chandra S, et al. A mitochondrial inside-out iron-calcium signal reveals drug targets for Parkinson’s disease. Cell Rep. 2023;42:113544.
Zhou X, Huang K, Wang Y, Zhang Z, Liu Y, Hou Q, et al. Evaluation of therapeutic effects of tetramethylpyrazine nitrone in Alzheimer’s disease mouse model and proteomics analysis. Front Pharm. 2023;14:1082602.
Li J, Li M, Ge Y, Chen J, Ma J, Wang C, et al. β-amyloid protein induces mitophagy-dependent ferroptosis through the CD36/PINK/PARKIN pathway leading to blood-brain barrier destruction in Alzheimer’s disease. Cell Biosci. 2022;12:69.
Igoillo-Esteve M, Gurgul-Convey E, Hu A, Romagueira Bichara Dos Santos L, Abdulkarim B, Chintawar S, et al. Unveiling a common mechanism of apoptosis in β-cells and neurons in Friedreich’s ataxia. Hum Mol Genet. 2015;24:2274–86.
Polishchuk EV, Merolla A, Lichtmannegger J, Romano A, Indrieri A, Ilyechova EY, et al. Activation of autophagy, observed in liver tissues from patients with Wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology. 2019;156:1173–89.
De Luna N, Turon-Sans J, Cortes-Vicente E, Carrasco-Rozas A, Illán-Gala I, Dols-Icardo O, et al. Downregulation of miR-335-5P in amyotrophic lateral sclerosis can contribute to neuronal mitochondrial dysfunction and apoptosis. Sci Rep. 2020;10:4308.
Salvany S, Casanovas A, Piedrafita L, Gras S, Calderó J, Esquerda JE. Accumulation of misfolded SOD1 outlines distinct patterns of motor neuron pathology and death during disease progression in a SOD1G93A mouse model of amyotrophic lateral sclerosis. Brain Pathol. 2022;32:e13078.
Wang D, Liang W, Huo D, Wang H, Wang Y, Cong C, et al. SPY1 inhibits neuronal ferroptosis in amyotrophic lateral sclerosis by reducing lipid peroxidation through regulation of GCH1 and TFR1. Cell Death Differ. 2023;30:369–82.
Vara-Pérez M, Rossi M, Van den Haute C, Maes H, Sassano ML, Venkataramani V, et al. BNIP3 promotes HIF-1α-driven melanoma growth by curbing intracellular iron homeostasis. EMBO J. 2021;40:e106214.
Qu F, Wang P, Zhang K, Shi Y, Li Y, Li C, et al. Manipulation of mitophagy by “All-in-One” nanosensitizer augments sonodynamic glioma therapy. Autophagy. 2020;16:1413–35.
Yin K, Lee J, Liu Z, Kim H, Martin DR, Wu D, et al. Mitophagy protein PINK1 suppresses colon tumor growth by metabolic reprogramming via p53 activation and reducing acetyl-CoA production. Cell Death Differ. 2021;28:2421–35.
Liu M, Fan Y, Li D, Han B, Meng Y, Chen F, et al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol Oncol. 2021;15:2084–105.
Wang Y, Zhu J, Liu Z, Shu S, Fu Y, Liu Y, et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. 2021;38:101767.
Yang C, Sun P, Deng M, Loughran P, Li W, Yi Z, et al. Gasdermin D protects against noninfectious liver injury by regulating apoptosis and necroptosis. Cell Death Dis. 2019;10:481.
Liu F, Yang Y, Peng W, Zhao N, Chen J, Xu Z, et al. Mitophagy-promoting miR-138-5p promoter demethylation inhibits pyroptosis in sepsis-associated acute lung injury. Inflamm Res. 2023;72:329–46.
Zhang H, Feng YW, Yao YM. Potential therapy strategy: targeting mitochondrial dysfunction in sepsis. Mil Med Res. 2018;5:41.
NaveenKumar SK, Hemshekhar M, Sharathbabu BN, Kemparaju K, Mugesh G, Girish KS. Platelet activation and ferroptosis mediated NETosis drives heme induced pulmonary thrombosis. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166688.
Shao Y, Saredy J, Xu K, Sun Y, Saaoud F, Drummer C IV, et al. Endothelial immunity trained by coronavirus infections, DAMP stimulations and regulated by anti-oxidant NRF2 may contribute to inflammations, myelopoiesis, COVID-19 cytokine storms and thromboembolism. Front Immunol. 2021;12:653110.
Li W, **ang Z, **ng Y, Li S, Shi S. Mitochondria bridge HIF signaling and ferroptosis blockage in acute kidney injury. Cell Death Dis. 2022;13:308.
Hu Z, Yuan Y, Zhang X, Lu Y, Dong N, Jiang X, et al. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate oxygen-glucose deprivation/reperfusion-induced microglial pyroptosis by promoting FOXO3a-dependent mitophagy. Oxid Med Cell Longev. 2021;2021:6219715.
Zhu H, Tan Y, Du W, Li Y, Toan S, Mui D, et al. Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol. 2021;38:101777.
Acknowledgements
Images were created using BioRender.com.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2022YFA1104604), and the National Natural Science Foundation of China (82241062, 82302412).
Author information
Authors and Affiliations
Contributions
Y-MY, R-QY and Y-PT conceptualized, supervised, and revised the manuscript. Q-YZ and CR drafted the manuscript. Q-YZ and LW illustrated the figures for the manuscript. J-YL and YD collected the relevant research. All authors approved the final manuscript and declared no competing financial interests.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Stephen Tait
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Qy., Ren, C., Li, Jy. et al. The crosstalk between mitochondrial quality control and metal-dependent cell death. Cell Death Dis 15, 299 (2024). https://doi.org/10.1038/s41419-024-06691-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06691-w
- Springer Nature Limited