Abstract
Intestinal stem cells (ISCs) play a crucial role in the continuous self-renewal and recovery of the intestinal epithelium. In previous studies, we have revealed that the specific absence of Claudin-7 (Cldn-7) in intestinal epithelial cells (IECs) can lead to the development of spontaneous colitis. However, the mechanisms by which Cldn-7 maintains homeostasis in the colonic epithelium remain unclear. Therefore, in the present study, we used IEC- and ISC-specific Cldn-7 knockout mice to investigate the regulatory effects of Cldn-7 on colonic Lgr5+ stem cells in the mediation of colonic epithelial injury and repair under physiological and inflammatory conditions. Notably, our findings reveal that Cldn-7 deletion disrupts the self-renewal and differentiation of colonic stem cells alongside the formation of colonic organoids in vitro. Additionally, these Cldn-7 knockout models exhibited heightened susceptibility to experimental colitis, limited epithelial repair and regeneration, and increased differentiation toward the secretory lineage. Mechanistically, we also established that Cldn-7 facilitates the proliferation, differentiation, and organoid formation of Lgr5+ stem cells through the maintenance of Wnt and Notch signalling pathways in the colonic epithelium. Overall, our study provides new insights into the maintenance of ISC function and colonic epithelial homoeostasis.
Similar content being viewed by others
Introduction
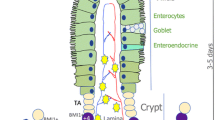
Adult stem cells play a pivotal role in maintaining homoeostasis and facilitating tissue repair through their capacity for self-renewal and differentiation. Notably, the intestinal epithelium possesses an enhanced capacity for self-renewal, occurring approximately every 4–5 days [1]. This rapid turnover is driven by intestinal stem cells (ISCs) located at the base of epithelial crypts [2]. Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5), a target gene in Wnt signalling, serves as a widely recognised marker for gastrointestinal stem cells [3]. Notably, +4 label-retaining cells are located specifically at the +4 position of the crypt, which is marked by the expression of Bmi1, Hopx, mTert, and Lrig1 [1, 4,5,6,7,8].
Stem cells give rise to transit-amplifying cells, which in turn differentiate into various cell lineages, including nutrient-absorbing enterocytes, protective mucus-secreting goblet cells, antimicrobial compound- and growth factor-producing Paneth cells, hormone-secreting enteroendocrine cells, and immune response-mediating chemosensory Tuft cells [9]. REG4/cKit-labelled deep crypt secretory cells in the colon are functionally similar to Paneth cells, providing specific support for Lgr5+ colonic stem cells [10, 11]. These cells migrate toward the intestinal lumen, undergo apoptosis, and are shed into the intestinal lumen, resulting in a continuous turnover of epithelial cells [12]. Lineage-tracing experiments have revealed that Lgr5+ ISCs can differentiate into all types of intestinal cells, form intact intestinal crypts, and produce organoids in vitro, which continuously maintain intestinal epithelial homoeostasis [3, 13].
The intestinal epithelium also rapidly regenerates in response to acute injury. Once damaged, the intestinal epithelium undergoes epithelial repair, in which ISCs activate, proliferate, and differentiate to restore the epithelium [14,15,16]. Acute inflammation has been shown to eradicate Lgr5+ ISCs in the small intestine and colon; this depletion of stem cells can delay or inhibit the regeneration of the damaged intestinal epithelium [17, 18]. Despite the robust regenerative capacity of ISCs, severe injury can disrupt intestinal epithelial homoeostasis, leading to inflammatory bowel disease (IBD), such as Crohn’s disease or ulcerative colitis (UC) [19, 20].
Maintaining the integrity of the intestinal barrier is crucial for host intestinal homoeostasis, which relies on the normal morphology and function of intestinal epithelial cells (IECs) and the formation of tight junctions [21, 22]. Claudin-7 (Cldn-7), a tight junction protein, is expressed in the apical, lateral, and basal membranes of IECs, with widespread expression in intestinal crypt stem cells [23]. Our previous research highlighted a significant down-regulation of Cldn-7 expression in UC; additionally, Cldn-7 deficiency led to spontaneous colitis, increasing susceptibility to dextran sulphate sodium (DSS)-induced epithelial injury [24]. Moreover, Cldn-7 has been found to contribute to the maintenance of ISCs homoeostasis in the small intestine [1.
Western blot analysis
Total protein was extracted from frozen colonic tissues using PIRA lysates supplemented with protease inhibitors; protein quantification was then performed using a BCA kit (Solaibao Life Science). Equal amounts of total protein were separated on 4–12% SDS-PAGE gels before being transferred to nitrocellulose membranes. the membranes were blocked with 5% non-fat dry milk and incubated with primary antibodies overnight at 4 °C. After washing with TBST, the membranes were incubated with secondary antibodies for 2 h at room temperature. Finally, the immunoreactive bands were imaged using the Ll-COR Odyssey Imaging System (LI-COR). Western blotting was repeated at least three times; representative images have been presented in corresponding figures. The antibodies used in western blotting are listed in Supplementary Table 2. All uncropped immunoblots are shown in Supplementary Fig. 6.
Statistical analyses
All experiments in this study were independently repeated a minimum of three times, with the number of animals in each experiment being specified in the figure legends. Data visualisation and statistical analyses were performed using GraphPad Prism 8.0.2 software. All data are presented as the mean ± standard error of the mean (SEM). Unpaired Student’s t-test (two-tailed) or nonparametric Mann–Whitney U test was used for comparisons between the two groups. Changes in body weight over time were analysed using repeated-measures ANOVA. Histopathological examination and statistical analyses were carried out by two authors blinded to experimental design. A p value < 0.05 was considered statistically significant.
Data availability
The authors declare that all data supporting the findings of this work are available within the main text and the supplementary information files, or from the corresponding author upon reasonable request.
References
Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33.
Santos AJM, Lo YH, Mah AT, Kuo CJ. The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol. 2018;28:1062–78.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7.
Muñoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S. et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012;31:3079–91.
Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–20.
Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–4.
Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE. et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA. 2011;108:179–84.
Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK. et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–58.
Beumer J, Clevers H. Cell fate specification and differentiation in the adult mammalian intestine. Nat Rev Mol Cell Biol. 2021;22:39–53.
Rothenberg ME, Nusse Y, Kalisky T, Lee JJ, Dalerba P, Scheeren F. et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology. 2012;142:1195–205.e6.
Sasaki N, Sachs N, Wiebrands K, Ellenbroek SI, Fumagalli A, Lyubimova A. et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci USA. 2016;113:E5399–407.
Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35–60.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
Wang Y, Chiang IL, Ohara TE, Fujii S, Cheng J, Muegge BD. et al. Long-term culture captures injury-repair cycles of colonic stem cells. Cell. 2019;179:1144–59.e15.
Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target?. Nat Rev Gastroenterol Hepatol. 2017;14:9–21.
Zheng L, Duan SL. Molecular regulation mechanism of intestinal stem cells in mucosal injury and repair in ulcerative colitis. World J Gastroenterol. 2023;29:2380–96.
Schmitt M, Schewe M, Sacchetti A, Feijtel D, van de Geer WS, Teeuwssen M, et al. Paneth cells respond to inflammation and contribute to tissue regeneration by acquiring stem-like features through SCF/c-Kit signaling. Cell Rep. 2018;24:2312–28.e7.
Davidson LA, Goldsby JS, Callaway ES, Shah MS, Barker N, Chapkin RS. Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta. 2012;1822:1600–7.
Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306.
Rees WD, Tandun R, Yau E, Zachos NC, Steiner TS. Regenerative intestinal stem cells induced by acute and chronic injury: the saving grace of the epithelium? Front Cell Dev Biol. 2020;8:583919.
Otani T, Furuse M. Tight junction structure and function revisited. Trends Cell Biol. 2020;30:805–17.
Garcia MA, Nelson WJ, Chavez N. Cell-cell junctions organize structural and signaling networks. Cold Spring Harb Perspect Biol. 2018;10:a029181.
Ding L, Lu Z, Foreman O, Tatum R, Lu Q, Renegar R. et al. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology. 2012;142:305–15.
Wang K, Ding Y, Xu C, Hao M, Li H, Ding L. Cldn-7 deficiency promotes experimental colitis and associated carcinogenesis by regulating intestinal epithelial integrity. Oncoimmunology. 2021;10:1923910.
**ng T, Benderman LJ, Sabu S, Parker J, Yang J, Lu Q. et al. Tight junction protein claudin-7 is essential for intestinal epithelial stem cell self-renewal and differentiation. Cell Mol Gastroenterol Hepatol. 2020;9:641–59.
Xu C, Ding YH, Wang K, Hao M, Li H, Ding L. Claudin-7 deficiency promotes stemness properties in colorectal cancer through Sox9-mediated Wnt/β-catenin signalling. J Transl Med. 2021;19:311.
Xu C, Wang K, Ding YH, Li WJ, Ding L. Claudin-7 gene knockout causes destruction of intestinal structure and animal death in mice. World J Gastroenterol. 2019;25:584–99.
van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE. et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–12.
Roche KC, Gracz AD, Liu XF, Newton V, Akiyama H, Magness ST. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology. 2015;149:1553–63.e10.
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–23.
Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–25.
Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells. 2012;30:741–52.
Tian H, Biehs B, Chiu C, Siebel CW, Wu Y, Costa M, et al. Opposing activities of Notch and Wnt signaling regulate intestinal stem cells and gut homeostasis. Cell Rep. 2015;11:33–42.
Li Y, Liu Y, Liu B, Wang J, Wei S, Qi Z. et al. A growth factor-free culture system underscores the coordination between Wnt and BMP signaling in Lgr5(+) intestinal stem cell maintenance. Cell Discov. 2018;4:49.
Wilson SS, Mayo M, Melim T, Knight H, Patnaude L, Wu X. et al. Optimized culture conditions for improved growth and functional differentiation of mouse and human colon organoids. Front Immunol. 2020;11:547102.
Wang Z, Qu YJ, Cui M. Modulation of stem cell fate in intestinal homeostasis, injury and repair. World J Stem Cells. 2023;15:354–68.
Rogers AP, Mileto SJ, Lyras D. Impact of enteric bacterial infections at and beyond the epithelial barrier. Nat Rev Microbiol. 2023;21:260–74.
Mennillo E, Yang X, Paszek M, Auwerx J, Benner C, Chen S. NCoR1 protects mice from dextran sodium sulfate-induced colitis by guarding colonic crypt cells from luminal insult. Cell Mol Gastroenterol Hepatol. 2020;10:133–47.
Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16:19–34.
Liu Y, Liu Z, Hu L, He L, Yang L, Qin Z. et al. Function of stem cells in radiation-induced damage. Int J Radiat Biol. 2023;99:1483–94.
Li N, Xu S, Zhang S, Zhu Q, Meng X, An W. et al. MSI2 deficiency in ILC3s attenuates DSS-induced colitis by affecting the intestinal microbiota. Front Immunol. 2022;13:963379.
Duckworth CA. Identifying key regulators of the intestinal stem cell niche. Biochem Soc Trans. 2021;49:2163–76.
Hageman JH, Heinz MC, Kretzschmar K, van der Vaart J, Clevers H, Snippert HJG. Intestinal regeneration: regulation by the microenvironment. Dev Cell. 2020;54:435–46.
Zhu Y, Li X. Advances of Wnt signalling pathway in colorectal cancer. Cells. 2023;12:447.
Mah AT, Yan KS, Kuo CJ. Wnt pathway regulation of intestinal stem cells. J Physiol. 2016;594:4837–47.
Liang SJ, Li XG, Wang XQ. Notch signaling in mammalian intestinal stem cells: determining cell fate and maintaining homeostasis. Curr Stem Cell Res Ther. 2019;14:583–90.
Kurokawa K, Hayakawa Y, Koike K. Plasticity of intestinal epithelium: stem cell niches and regulatory signals. Int J Mol Sci. 2020;22:357.
Li WJ, Xu C, Wang K, Li TY, Wang XN, Yang H. et al. Severe intestinal inflammation in the small intestine of mice induced by controllable deletion of claudin-7. Dig Dis Sci. 2018;63:1200–9.
Tirado FR, Bhanja P, Castro-Nallar E, Olea XD, Salamanca C, Saha S. Radiation-induced toxicity in rectal epithelial stem cell contributes to acute radiation injury in rectum. Stem Cell Res Ther. 2021;12:63.
Acknowledgements
We would like to thank Professor Yong Tian (Institute of Biophysics, Chinese Academy of Sciences) for the kind gift of Lgr5-EGFP-IRES-CreERT2 mice. We thank Yu Luo and Pengfei Wu for the advice and technical assistance. This work was supported by grants from the National Natural Science Foundation of China (Grant # 82170525) and Bei**g Natural Science Foundation (Grant # 7192087).
Author information
Authors and Affiliations
Contributions
K.W. and Y.L. designed performed the experiments and wrote the manuscript; K.W. generated and characterised the knockout mice; H.M.L. and X.Q.L. assisted with mouse intraperitoneal injection and organoid culture; Y.L. and M.D.H. provided technical assistance with RNA-seq data analysis; D.J.Y. contributed to histological staining and analysis; L.D. led the conceptualisation and provided valuable infrastructure, guidance, and additional funding for the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures involving animals were followed the ARRIVE guidelines and approved by the Animal Ethics Committee of Bei**g Shijitan Hospital Institutional Review Board (sjtkyll-1x-2021(105)).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Stephen Tait
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, K., Liu, Y., Li, H. et al. Claudin-7 is essential for the maintenance of colonic stem cell homoeostasis via the modulation of Wnt/Notch signalling. Cell Death Dis 15, 284 (2024). https://doi.org/10.1038/s41419-024-06658-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06658-x
- Springer Nature Limited