Abstract
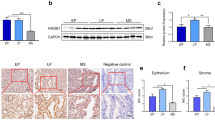
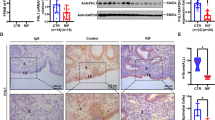
Infertility occurs in 15% of couples worldwide. Recurrent implantation failure (RIF) is one of the major problems in in vitro fertilization and embryo transfer (IVF–ET) programs, and how to manage patients with RIF to achieve successful pregnancy outcomes remains unresolved. Here, a uterine polycomb repressive complex 2 (PRC2)-regulated gene network was found to control embryo implantation. Our RNA-seq analyses of the human peri-implantation endometrium obtained from patients with RIF and fertile controls revealed that PRC2 components, including its core enzyme enhancer of zeste homolog 2 (EZH2)-catalyzing H3K27 trimethylation (H3K27me3) and their target genes are dysregulated in the RIF group. Although fertility of uterine epithelium-specific knockout mice of Ezh2 (eKO mice) was normal, Ezh2-deleted mice in the uterine epithelium and stroma (uKO mice) exhibited severe subfertility, suggesting that stromal Ezh2 plays a key role in female fertility. The RNA-seq and ChIP-seq analyses revealed that H3K27me3-related dynamic gene silencing is canceled, and the gene expression of cell-cycle regulators is dysregulated in Ezh2-deleted uteri, causing severe epithelial and stromal differentiation defects and failed embryo invasion. Thus, our findings indicate that the EZH2–PRC2–H3K27me3 axis is critical to preparing the endometrium for the blastocyst invasion into the stroma in mice and humans.
Similar content being viewed by others
Introduction
Appropriate communication between the fetus and endometrium is critical for healthy pregnancy outcomes [1, 2]. Infertility is an important social concern encountered by approximately 15% of couples during their reproductive age worldwide [3]. In vitro fertilization and embryo transfer (IVF–ET) have been developed in the last 40 years, providing healthy births to infertile couples. However, by improving the techniques to select good-quality sperms, oocytes, and fertilized embryos, only 30% of ETs succeed in having pregnancies [2]. Recurrent implantation failure (RIF) is one of the major issues in IVF–ET programs. Therefore, an inappropriate endometrial condition is believed to be a critical factor to cause RIF [1]; however, how the endometrial dysfunction causes RIF remains unclear.
Embryo implantation is a complex process of communication between blastocysts and endometria, molecularly and physically [1, 4]. Due to similarities in hormonal cycles and the manner of pregnancy maintenance, rodents are often used as a model to analyze embryo implantation processes as an alternative to human studies [1, 2, 4]. After coitus, ovaries provide increasing progesterone (P4) levels to prepare the endometria receptive to blastocysts [5]. With the influence of P4, the endometrial epithelium stops growing and begins to differentiate [6, 1]. Embryo implantation anomalies have been reported to severely affect pregnancy maintenance spanning species including humans [2]. Most of the studies using KO mice have shown that several single molecules influence the implantation and following pregnancy conditions, whereas their impacts in humans remain largely uncertain [1, 4]. The recent development of next-generation sequencing found that gene expressions are dynamically altered during menstrual cycles, possibly contributing to endometrial regenerations and implantation window opening [37, 38]. Although this beneficial information regarding menstrual tissues has already been reported, it remains unclear how gene expression signatures differ between endometria with successful and failed implantation. In our study, we dissected DEGs in human peri-implantation endometrium obtained from RIF and fertile patients. Enrichments of PRC2–H3K27me3-targeting genes were evident in DEGs, implying the possible roles of this axis to regulate genome-wide expressions during embryo implantation. Furthermore, the essential roles of Ezh2 in early pregnancy were identified by applying next-generation sequencing in Ezh2 uKO endometria. Previously, Nanjappa et al. and Fang et al. reported that Ezh2 uKO uteri exhibit abnormal epithelial integrities in their old ages; however, detailed mechanisms were not shown [27, 28]. They also compared gene expressions between the control and Ezh2 uKO under the treatment of exogenous P4 and E2, which might explain increased epithelial cell expansions in this milieu [39]. Recently, Osokine et al. investigated how Ezh2 contributes to decidual functions upon wound healing [40], focusing on post-implantation events after day 8 of implantation when deciduae were already terminally differentiated [48]. PRC1-induced H2AK119u1 can recruit PRC2 to promote H3K27 methylations [47]. As pharmaceutical inhibitions of either DNA methylations or PRC1 resulted in terminations of pregnancy in mice [49, 50], these epigenetic regulations may prepare uterine genomes for the following PRC2 binding. We also have not yet assessed how PRC2-induced H3K27me3 is canceled during the pregnancy progression. A report demonstrated that uterine tissues exhibit acetylation on lysines 4 and 27 of histone H3, the two major active histone markers, upon parturition [51]. This implies that some system works to determine pregnancy stages, switching epigenetic modifications. Future endeavors should examine how uterine cells respond to pregnancy conditions with appropriate gene expressions at each pregnancy condition.
Methods
Collection of human endometrial tissues in the peri-implantation period
Human endometrial tissues in the peri-implantation period were obtained from patients undergoing IVF–ET treatment aged under 40 years. Endometrial biopsy was performed as previously described [20]. The specimens obtained from those with uterine fibroids, adenomyosis, endometrial polyps, endometrial hyperplasia, and endometriosis were excluded from the study because these diseases have the possibility to affect endometrial receptivity. To minimize individual differences in hormonal status, the same protocol of hormonal replacement cycle for frozen ET was used for all patients in the cycle of endometrial biopsy. Endometrial biopsies were performed on day 7 of P4 administration during a hormonal replacement cycle, which is considered the peri-implantation period in humans. Patients underwent ET in subsequent cycles after the endometrial biopsy, and the outcome of clinical pregnancy was monitored. RIF patients were defined as those who had more than two failed embryo transfer cycles using good-quality embryos. The fertile controls were defined as patients who had clinical pregnancy in the subsequent cycle after the endometrial biopsy. Twelve and 26 independent endometrial samples were collected from patients with RIF and fertile controls, respectively. Table 1 demonstrated the information of the patients with RIF and the fertile controls. The study protocols using human specimens were approved by the institutional review board of the University of Tokyo (IRB numbers: 10991 and 2019241 G), and each woman signed informed consent for the use of tissues.
RNA-seq of the human endometrium
RNA extraction from human endometrial tissues was performed using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). RNA-seq was performed at Macrogen Japan (Tokyo, Japan) on 38 specimens: 26 patients clinically pregnant as a result of embryo transfers after endometrial biopsy (fertile controls; successful implantation group) and 12 who did not become clinically pregnant (the RIF group; failed implantation group). The resulting raw read files were aligned on the human genome sequence (GRCh38/hg38) using the Hisat2 version 2.1.0 [52], and the number of reads at each locus was counted using the featureCount function [53] in the Subread tool. To compare expression levels between successful and failed implantation samples, read count files were submitted to DESeq2 version 1.16.1 [54]. DEGs were defined as genes showing >twofold difference in expression levels with a significant difference of P < 0.5 (P-values were adjusted for multiple testing using the Benjamini–Hochberg method). Moreover, these genes proceeded to enrichment analyses in Enrichr (https://maayanlab.cloud/Enrichr/) [62]. Table S1 shows qPCR primer sequences used to detect each gene.
Three-dimensional visualization of ISs
As previously reported, three-dimensional (3D) visualization on day 6 ISs was performed [9, 60]. Briefly, an anti-E-cadherin antibody (3195, Cell Signaling Technology, 1:300) was used to stain luminal and glandular epithelia. Alexa Fluor 555-conjugated anti-rabbit antibody (A21428, Thermo Fisher Scientific, 1:300) was used as a secondary antibody. The 3D images were acquired using LSM 800 (Zeiss, Oberkochen, Germany) and AXR (Nikon, Tokyo, Japan). The surface tool in Imaris (v 9.8, Oxford Instruments, Abingdon-on-Thames, UK) was used to construct 3D images.
Native ChIP
Day 6 uteri from the control and Ezh2 uKO were used. Uterine tissues surrounding the embryos were longitudinally opened at the mesometrial side and kept at −80 °C until use. Endometrial tissues were disrupted with 35 strokes in a Dounce homogenizer on ice, with a loose-fitting pestle in PBS-containing protease inhibitor cocktail and phosphatase inhibitors 2 and 3 (Sigma, St. Louis, MO, USA). After centrifugation with 1000g for 5 min, pellets were received in the nuclei EZ lysis buffer (Sigma) to isolate the nuclei. Native ChIP was then performed as previously described [63], with some modifications. For the fragmentation, chromatins were treated by 20 U/μl MNase at 37 °C for 5 min. Input DNA was analyzed with a 2100 Bioanalyzer system using High-Sensitivity DNA Reagent kit (Agilent, Santa Clara, CA, USA) to confirm DNA fragmentations at approximately 200–300 bps. Chromatin immunoprecipitation was performed using Magna ChIP G-Chromatin Immunoprecipitation Kit (Millipore, Burlington, MA, USA) following the manufacturer’s protocol. Anti-H3K27me3 antibody (39155, Active motif, Carlsbad, CA, USA) or anti-rabbit IgG (2729, Cell Signaling Technology) was used to precipitate the target chromatins. Chromatin-immunoprecipitated DNAs were purified by extracting phenol–chloroforms.
ChIP-seq
ChIP samples were subjected to ChIP-seq using Novogene Inc. service. The resulting raw read files were aligned on the mouse genome sequence (GRCm38/mm10) using bowtie2 version 2.3.3.1, and peak cells were made using MACS2 [64] with their default settings. Tag density plots and heatmaps in the vicinity were generated using ngs.plot version 2.47.1 [65]. BEDtools intersect [66] (version 2.26.0) was used to compute the numbers of H3K27me3 peaks within ±2 kb around the upregulated genes in Ezh2 uKO on day 6. Enrichment of H3K27me3 peaks around the target loci was then compared between the control and Ezh2 uKO with Mann–Whitney U test using R (4.0.2). For the analysis focusing on G2M-related cell cycle genes, human cell cycle genes previously reported [67] were converted into mouse gene names using biomaRt in R (4.0.2). To identify the enrichment of known motifs within H3K27me3 enriched loci, we used the HOMER [68] (version 4.9) function findMotifsGenome.pl with default parameters and a fragment size denoted by the argument -gain. To visualize H3K27me3 peaks, the IGVTools count function [69] (Broad Institute, Cambridge, MA) was used to create TDF files from the sorted BAM files. The TDF files were processed in the IGV browser [69] (Broad Institute) to show continuous tag counts over the target loci.
In vivo bromo-deoxyuridine (BrdU) incorporation assay
In vivo BrdU incorporation assay was performed as previously described [70]. Briefly, female mice were injected with BrdU (100 mg per kg body weight) at 1000 h on day 6 of pregnancy. Two hours later, they were sacrificed and the uteri were frozen immediately. Frozen sections (12 μm) were fixed in methanol for 10 min at room temperature and immersed in 2 N HCl for 20 min at 37 °C to denature DNA for immunohistochemical detection. BrdU-positive area was quantified using Image J (NIH).
Immunofluorescence
Frozen sections (12 μm) were used for immunofluorescence. BrdU (ab6326, Abcam, 1:250) and phospho-histone H3 (pHH3) (9701, Cell Signaling Technology, 1:300) antibodies were used as primary antibodies. Alexa Fluor 488-conjugated anti-rat immunoglobulin G (A11006, Thermo Fisher Scientific, 1:300) and Alexa Fluor 555-conjugated anti-rabbit immunoglobulin G (A21428, Thermo Fisher Scientific, 1:300) were used for signal detection with nuclear staining using 4,6-diamidino-2-phenylindole (Do**do, Kumamoto, Japan 1:500). Images were obtained using LSM 800 (Zeiss) and AXR (Nikon). The decidual area without pHH3 staining was quantified by Image J (NIH).
Senescence-associated β-galactosidase (SAβgal) staining
To evaluate the terminal differentiation of decidua, SAβgal activity staining was performed as previously described [35, 36]. To compare the intensity of SAβgal staining between the control and Ezh2 uKO, frozen sections from both genotypes were processed on the same slide. Sections were counterstained with eosin.
Statistical analysis
Statistical analyses were performed using two-tailed Student’s t-test in GraphPad Prism 9 (GraphPad Software, San Diego, CA); otherwise, they are described in detail in each experimental section.
Data availability
The data of RNA-seq and ChIP-seq were deposited in Gene Expression Omnibus (The accession number: GSE207362). Among the uploaded human RNA-seq data, we only used the one from patients aged under 40 years without uterine diseases: #2, 3, 5–7, 9–13, 16–18, 20, 21, 23, 24, 26, 31–33, 35, 36, and 38–40 for fertile control; #1, 4–7, 9, 14, 17, 19, 21, 23, and 25 for RIF.
References
Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–67.
Fukui Y, Hirota Y, Matsuo M, Gebril M, Akaeda S, Hiraoka T, et al. Uterine receptivity, embryo attachment, and embryo invasion: multistep processes in embryo implantation. Reprod Med Biol. 2019;18:234–40.
Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37.
Maurya VK, DeMayo FJ, Lydon JP. Illuminating the “Black Box” of progesterone-dependent embryo implantation using engineered mice. Front Cell Dev Biol. 2021;9:640907.
Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–90.
Aikawa S, Deng W, Liang X, Yuan J, Bartos A, Sun X, et al. Uterine deficiency of high-mobility group box-1 (HMGB1) protein causes implantation defects and adverse pregnancy outcomes. Cell Death Differ. 2020;27:1489–1504.
Daikoku T, Cha J, Sun X, Tranguch S, **e HR, Fujita T, et al. Conditional deletion of MSX homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Develop Cell. 2011;21:1014–25.
Kelleher AM, Milano-Foster J, Behura SK, Spencer TE. Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success. Nat Commun. 2018;9:2435.
Yuan J, Deng W, Cha J, Sun X, Borg JP, Dey SK. Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat Commun. 2018;9:603.
Sroga JM, Ma X, Das SK. Developmental regulation of decidual cell polyploidy at the site of implantation. Front Biosci (Sch Ed). 2012;4:1475–86.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–54. Suppl
Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21.
Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–19.
Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–9.
Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell. 2004;15:57–67.
Aikawa S, Hirota Y, Fukui Y, Ishizawa C, IIda R, Kaku T, et al. A gene network of uterine luminal epithelium organizes mouse blastocyst implantation. Reprod Med Biol. 2022;22:e12435.
Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–64.
Psychoyos A. Endocrine control of egg implantation. American Physiology Society: Washington, DC, 1973.
Matsumoto L, Hirota Y, Saito-Fujita T, Takeda N, Tanaka T, Hiraoka T, et al. HIF2α in the uterine stroma permits embryo invasion and luminal epithelium detachment. J Clin Invest. 2018;128:3186–97.
Akaeda S, Hirota Y, Fukui Y, Aikawa S, Shimizu-Hirota R, Kaku T, et al. Retinoblastoma protein promotes uterine epithelial cell cycle arrest and necroptosis for embryo invasion. EMBO Rep. 2021;22:e50927.
Li Y, Sun X, Dey SK. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 2015;11:358–65.
Arora R, Fries A, Oelerich K, Marchuk K, Sabeur K, Giudice LC, et al. Insights from imaging the implanting embryo and the uterine environment in three dimensions. Development. 2016;143:4749–54.
Hirota Y. Progesterone governs endometrial proliferation-differentiation switching and blastocyst implantation. Endocr J. 2019;66:199–206.
Wetendorf M, Wu SP, Wang X, Creighton CJ, Wang T, Lanz RB, et al. Decreased epithelial progesterone receptor A at the window of receptivity is required for preparation of the endometrium for embryo attachment. Biol Reprod. 2017;96:313–26.
**n Q, Kong S, Yan J, Qiu J, He B, Zhou C, et al. Polycomb subunit BMI1 determines uterine progesterone responsiveness essential for normal embryo implantation. J Clin Invest. 2018;128:175–89.
Gao F, Bian F, Ma X, Kalinichenko VV, Das SK. Control of regional decidualization in implantation: Role of FoxM1 downstream of Hoxa10 and cyclin D3. Sci Rep. 2015;5:13863.
Fang X, Ni N, Lydon JP, Ivanov I, Bayless KJ, Rijnkels M, et al. Enhancer of zeste 2 polycomb repressive complex 2 subunit is required for uterine epithelial integrity. Am J Pathol. 2019;189:1212–25.
Nanjappa MK, Mesa AM, Medrano TI, Jefferson WN, DeMayo FJ, Williams CJ, et al. The histone methyltransferase EZH2 is required for normal uterine development and function in micedagger. Biol Reprod. 2019;101:306–17.
Yuan J, Aikawa S, Deng W, Bartos A, Walz G, Grahammer F, et al. Primary decidual zone formation requires Scribble for pregnancy success in mice. Nat Commun. 2019;10:5425.
Li X, Lin Y, Yang X, Wu X, He X. Long noncoding RNA H19 regulates EZH2 expression by interacting with miR-630 and promotes cell invasion in nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2016;473:913–19.
Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–21.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6.
Das SK. Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction. 2009;137:889–99.
Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–66.
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67.
Hirota Y, Daikoku T, Tranguch S, **e H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest. 2010;120:803–15.
Wang W, Vilella F, Alama P, Moreno I, Mignardi M, Isakova A, et al. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat Med. 2020;26:1644–53.
Garcia-Alonso L, Handfield LF, Roberts K, Nikolakopoulou K, Fernando RC, Gardner L, et al. Map** the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet. 2021;53:1698–711.
Mesa AM, Mao J, Medrano TI, Bivens NJ, Jurkevich A, Tuteja G, et al. Spatial transcriptomics analysis of uterine gene expression in enhancer of zeste homolog 2 conditional knockout micedagger. Biol Reprod. 2021;105:1126–39.
Osokine I, Siewiera J, Rideaux D, Ma S, Tsukui T, Erlebacher A. Gene silencing by EZH2 suppresses TGF-beta activity within the decidua to avert pregnancy-adverse wound healing at the maternal-fetal interface. Cell Rep. 2022;38:110329.
Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–13.
Tavares M, Khandelwal G, Mutter J, Viiri K, Beltran M, Brosens JJ, et al. JAZF1-SUZ12 dysregulates PRC2 function and gene expression during cell differentiation. Cell Rep. 2022;39:110889.
Koontz JI, Soreng AL, Nucci M, Kuo FC, Pauwels P, van Den Berghe H, et al. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci USA. 2001;98:6348–53.
Lv S, Wang N, Lv H, Yang J, Liu J, Li WP, et al. The attenuation of trophoblast invasion caused by the downregulation of EZH2 is involved in the pathogenesis of human recurrent miscarriage. Mol Ther Nucleic Acids. 2019;14:377–87.
Pal B, Bouras T, Shi W, Vaillant F, Sheridan JM, Fu N, et al. Global changes in the mammary epigenome are induced by hormonal cues and coordinated by Ezh2. Cell Rep. 2013;3:411–26.
Reddington JP, Perricone SM, Nestor CE, Reichmann J, Youngson NA, Suzuki M, et al. Redistribution of H3K27me3 upon DNA hypomethylation results in de-repression of Polycomb target genes. Genome Biol. 2013;14:R25.
Cooper S, Dienstbier M, Hassan R, Schermelleh L, Sharif J, Blackledge NP, et al. Targeting polycomb to pericentric heterochromatin in embryonic stem cells reveals a role for H2AK119u1 in PRC2 recruitment. Cell Rep. 2014;7:1456–70.
Simon JA, Kingston RE. Occupying chromatin: polycomb mechanisms for getting to genomic targets, stop** transcriptional traffic, and staying put. Mol Cell. 2013;49:808–24.
Bian F, Gao F, Kartashov AV, Jegga AG, Barski A, Das SK. Polycomb repressive complex 1 controls uterine decidualization. Sci Rep. 2016;6:26061.
Gao F, Ma X, Rusie A, Hemingway J, Ostmann AB, Chung D, et al. Epigenetic changes through DNA methylation contribute to uterine stromal cell decidualization. Endocrinology. 2012;153:6078–90.
Shchuka VM, Abatti LE, Hou H, Khader N, Dorogin A, Wilson MD, et al. The pregnant myometrium is epigenetically activated at contractility-driving gene loci prior to the onset of labor in mice. PLoS Biol. 2020;18:e3000710.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60.
Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
**e Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, et al. Gene set knowledge discovery with enrichr. Curr Protoc. 2021;1:e90.
Hirabayashi Y, Suzki N, Tsuboi M, Endo TA, Toyoda T, Shinga J, et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron. 2009;63:600–13.
Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66.
Daikoku T, Ogawa Y, Terakawa J, Ogawa A, DeFalco T, Dey SK. Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology. 2014;155:2718–24.
Theiler K. The house mouse: atlas of embryonic development. Springer: Berlin, Heidelberg, 1989.
Fukui Y, Hirota Y, Saito-Fujita T, Aikawa S, Hiraoka T, Kaku T, et al. Uterine epithelial LIF receptors contribute to implantation chamber formation in blastocyst attachment. Endocrinology. 2021;162:bqab169.
Haraguchi H, Saito-Fujita T, Hirota Y, Egashira M, Matsumoto L, Matsuo M, et al. MicroRNA-200a locally attenuates progesterone signaling in the cervix, preventing embryo implantation. Mol Endocrinol. 2014;28:1108–17.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Sakashita A, Maezawa S, Takahashi K, Alavattam KG, Yukawa M, Hu YC, et al. Endogenous retroviruses drive species-specific germline transcriptomes in mammals. Nat Struct Mol Biol. 2020;27:967–77.
Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9:R137.
Shen L, Shao N, Liu X, Nestler E. ngs.plot: Quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics. 2014;15:284.
Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–2.
Viner-Breuer R, Yilmaz A, Benvenisty N, Goldberg M. The essentiality landscape of cell cycle related genes in human pluripotent and cancer cells. Cell Div. 2019;14:15.
Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–89.
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–6.
Aikawa S, Kano K, Inoue A, Wang J, Saigusa D, Nagamatsu T, et al. Autotaxin-lysophosphatidic acid-LPA3 signaling at the embryo-epithelial boundary controls decidualization pathways. EMBO J. 2017;36:2146–60.
Acknowledgements
We thank the RIKEN BioResource Research Center (the National Bio Resource Project of the MEXT, Japan) and Dr. Haruhiko Koseki (RIKEN, Japan) for providing Ezh2-floxed mice, Dr. Francesco J. DeMayo (National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA) and Dr. John P. Lydon (Baylor College of Medicine, Houston, TX, USA) for providing Pgr-Cre mice, Dr. Sudhansu K. Dey (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA) for providing Ltf-iCre mice, and Dr. Tomoki Tanaka and Ms. Atsumi Miura for technical assistance. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI (grant Nos. JP19H03144, JP19H03796, JP19K16022, JP21K16763, JP21J00509, JP22K16852, JP22H02538, JP22H03222, JP22K19595, and JP22K19596), Japan Agency for Medical Research and Development (AMED) Frontier Outstanding Research for Clinical Empowerment (FORCE) (grant No. JP21gm4010010), AMED Whole Implementation to Support and Ensure the Female Life (WISE) (grant Nos. JP 21gk0210021 and JP22gk0210028), AMED Project for Baby and Infant in Research of Health and Development to Adolescent and Young Adult (BIRTHDAY) (grant Nos. JP22gk0110056 and JP22gk0110069), AMED Research Project for Improving Quality in Healthcare and Collecting Scientific Evidence on Integrative Medicine (grant No. JP22lk0310083), Japan Science and Technology Agency (JST) Fusion Oriented Research for disruptive Science and Technology (FOREST) (grant No. JPMJFR210H), Mochida Memorial Foundation for Medical and Pharmaceutical Research, Uehara Memorial Foundation, Inoue Foundation for Science, and the fund of joint research with NIPRO corporation.
Author information
Authors and Affiliations
Contributions
YH and SAi designed the study. YF, YH, SAi, AS, CI, RI, TK, ToH, TaH, SAK, and MM performed experiments and collected the data. YF, YH, SAi, and AS analyzed the data. YF, YH, SAi, AS, RSH, NT, and YO discussed and interpreted the results. SAi drafted the paper, which was edited by YF and YH. RSH critically reviewed the paper. YH supervised the study. YF, YH, and SAi contributed equally to this work and are listed according to their relative contributions to the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fukui, Y., Hirota, Y., Aikawa, S. et al. The EZH2–PRC2–H3K27me3 axis governs the endometrial cell cycle and differentiation for blastocyst invasion. Cell Death Dis 14, 320 (2023). https://doi.org/10.1038/s41419-023-05832-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-023-05832-x
- Springer Nature Limited
This article is cited by
-
H3K27me3 timely dictates uterine epithelial transcriptome remodeling and thus transformation essential for normal embryo implantation
Cell Death & Differentiation (2024)